Similar presentations:
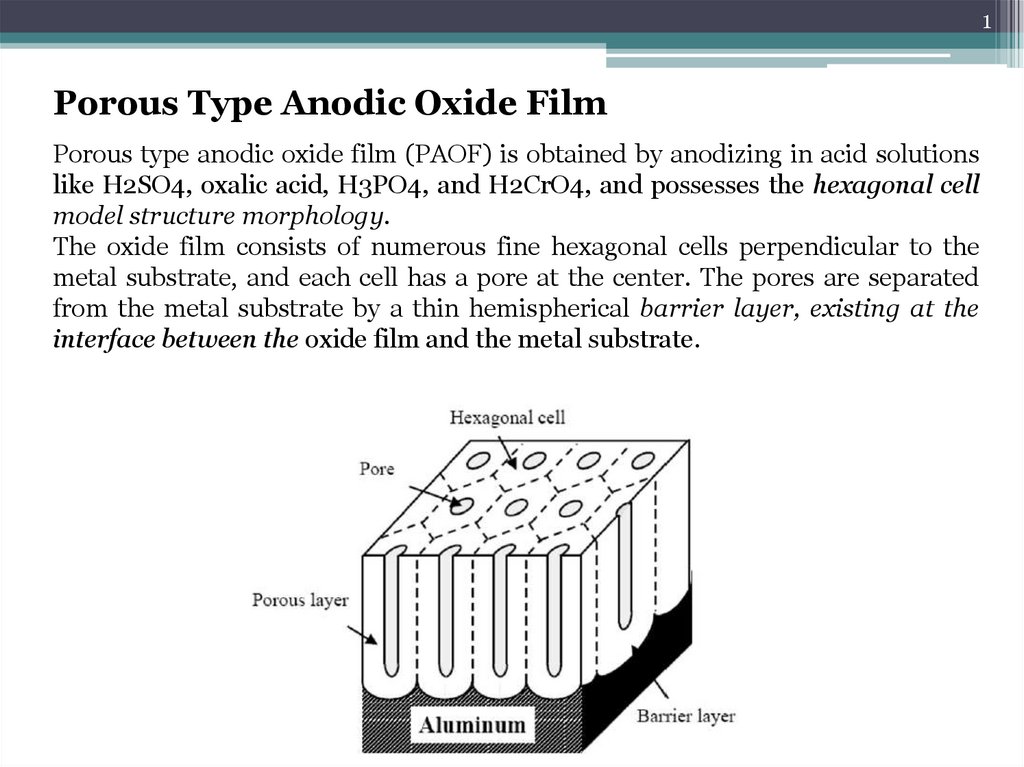
Porous Type Anodic Oxide Film
1.
1Porous Type Anodic Oxide Film
Porous type anodic oxide film (PAOF) is obtained by anodizing in acid solutions
like H2SO4, oxalic acid, H3PO4, and H2CrO4, and possesses the hexagonal cell
model structure morphology.
The oxide film consists of numerous fine hexagonal cells perpendicular to the
metal substrate, and each cell has a pore at the center. The pores are separated
from the metal substrate by a thin hemispherical barrier layer, existing at the
interface between the oxide film and the metal substrate.
2.
2Nanoporous anodic alumina was discovered during the first decades of the
twentieth century and widely used in industry for:
•corrosion protection,
•car industry,
•metal decoration purposes,
•optics and photonics,
•electronics,
•membrane science,
•materials science,
•engineering,
•medicine,
•industry.
More than 3000 journal papers on nanoporous anodic alumina were published in
last 20 years.
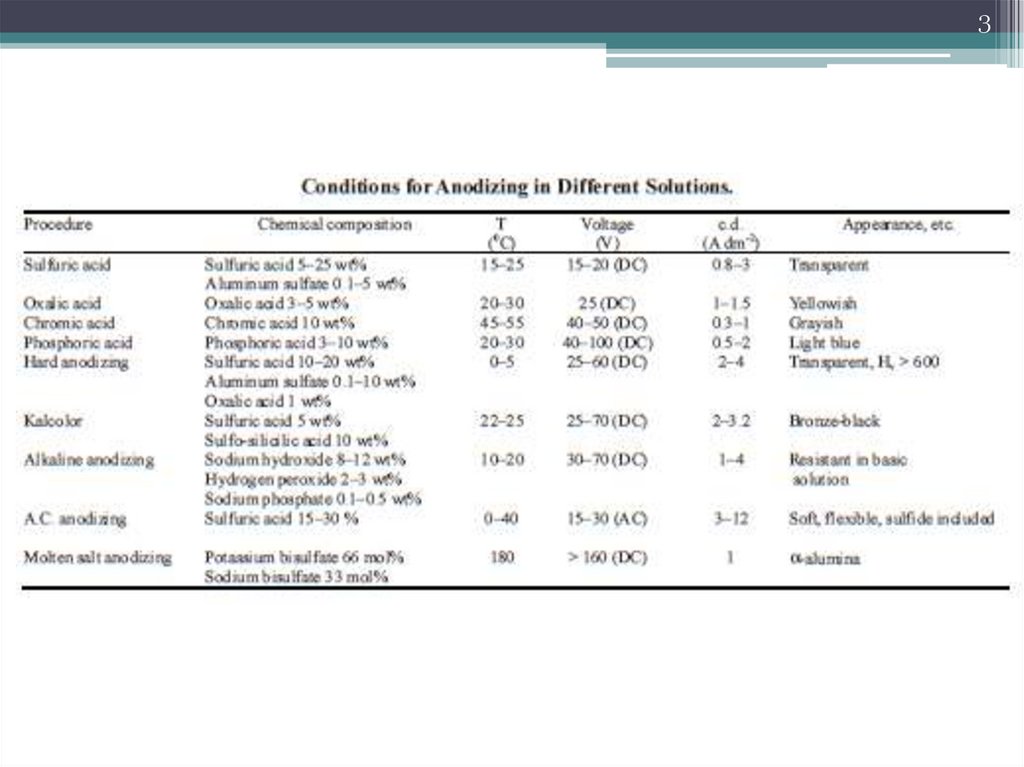
3.
34.
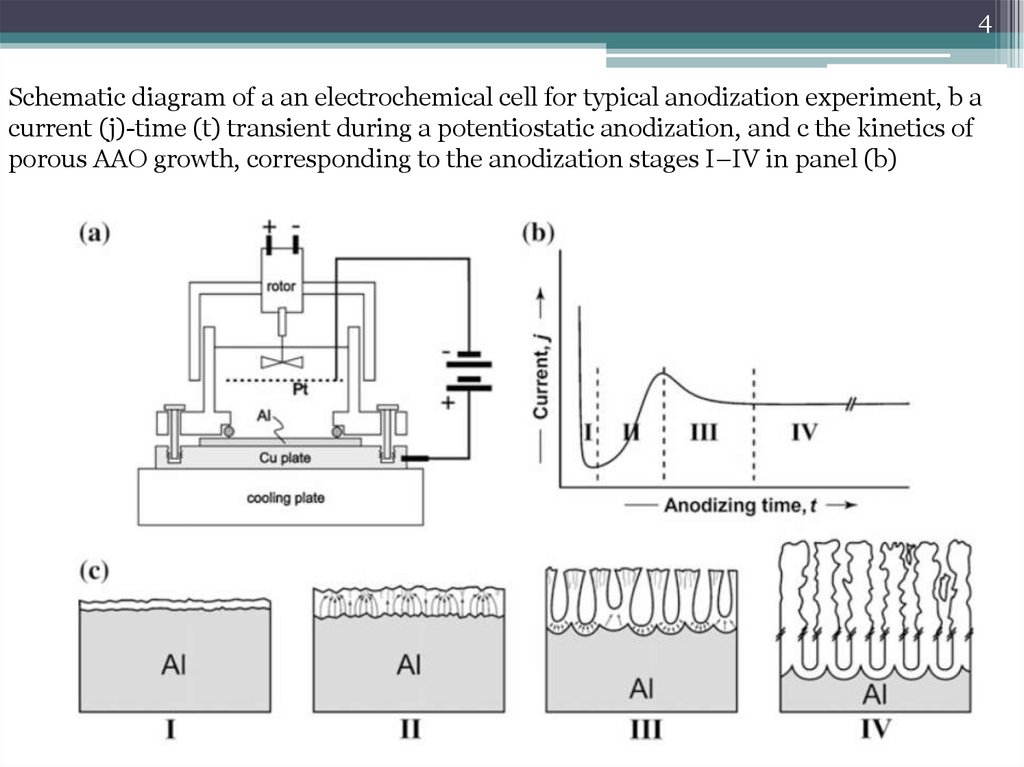
4Schematic diagram of a an electrochemical cell for typical anodization experiment, b a
current (j)-time (t) transient during a potentiostatic anodization, and c the kinetics of
porous AAO growth, corresponding to the anodization stages I–IV in panel (b)
5.
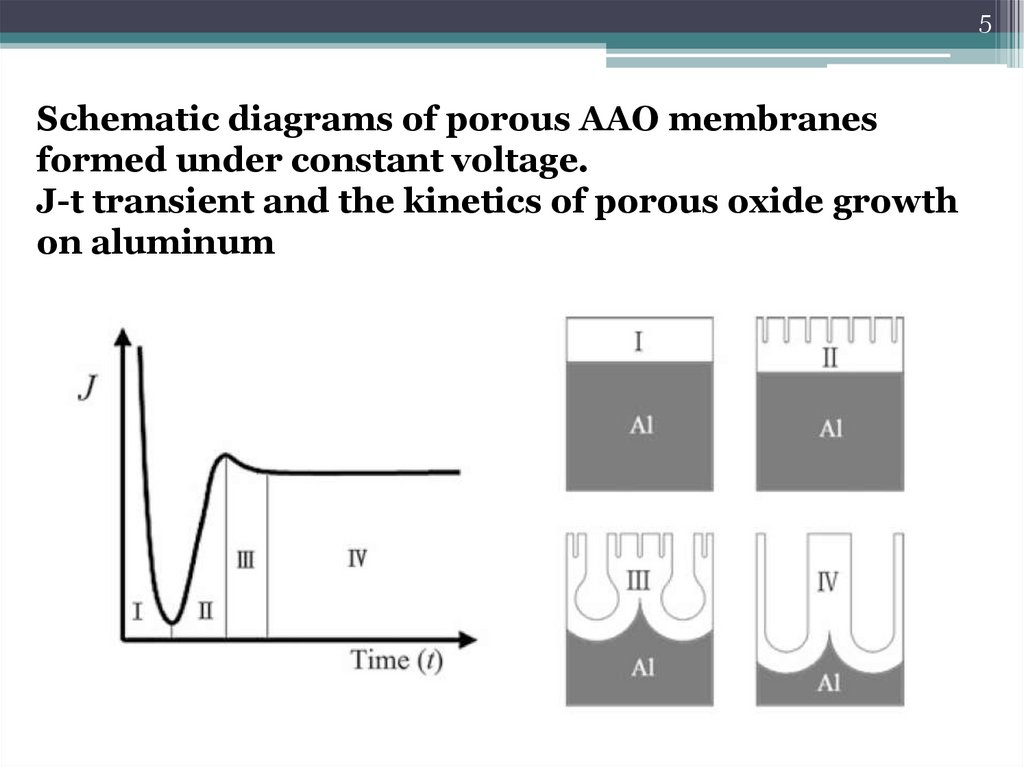
5Schematic diagrams of porous AAO membranes
formed under constant voltage.
J-t transient and the kinetics of porous oxide growth
on aluminum
6.
6Stage I
At the moment after applying a constant U, the J will reach a
high value quickly, which can be attributed to the occurrence of
electrolytic process of water.
Immediately, a thin compact oxide barrier layer begins to form
on the aluminum surface which in contact with the electrolyte.
At this stage, the thickness of the oxide barrier layer increases
rapidly, which means that the total resistance will increase
correspondingly. Therefore, the J will decrease abruptly to
reach the minimum value under the potentiostatic mode, when
the barrier layer thickness increases to a certain value
7.
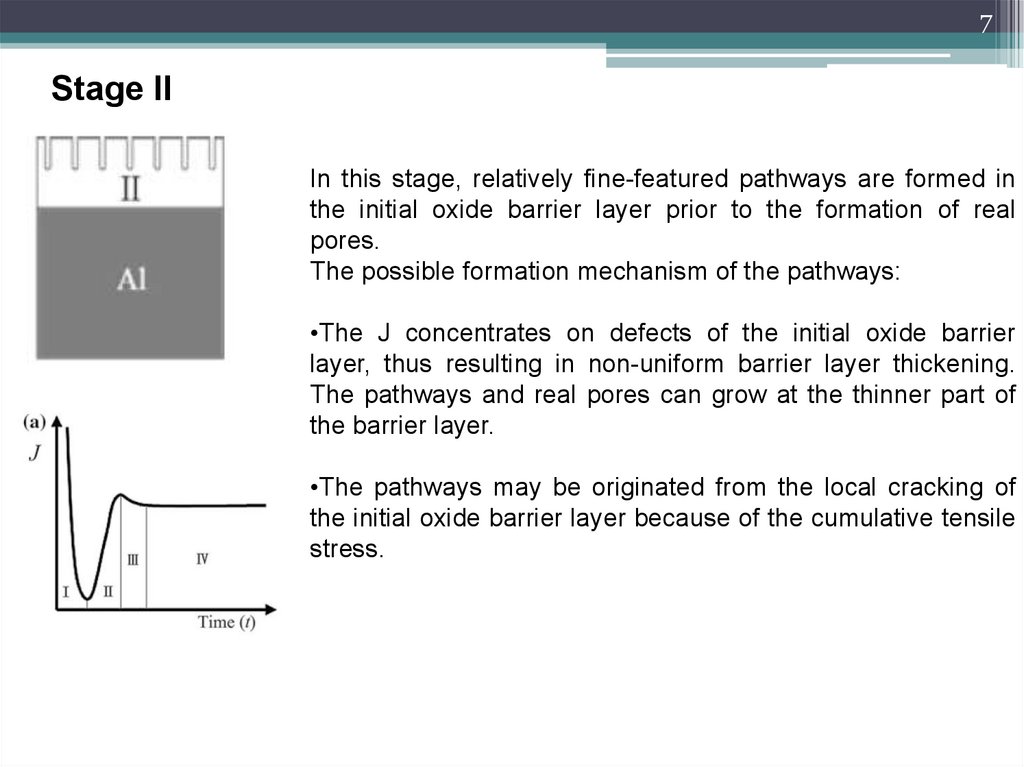
7Stage II
In this stage, relatively fine-featured pathways are formed in
the initial oxide barrier layer prior to the formation of real
pores.
The possible formation mechanism of the pathways:
•The J concentrates on defects of the initial oxide barrier
layer, thus resulting in non-uniform barrier layer thickening.
The pathways and real pores can grow at the thinner part of
the barrier layer.
•The pathways may be originated from the local cracking of
the initial oxide barrier layer because of the cumulative tensile
stress.
8.
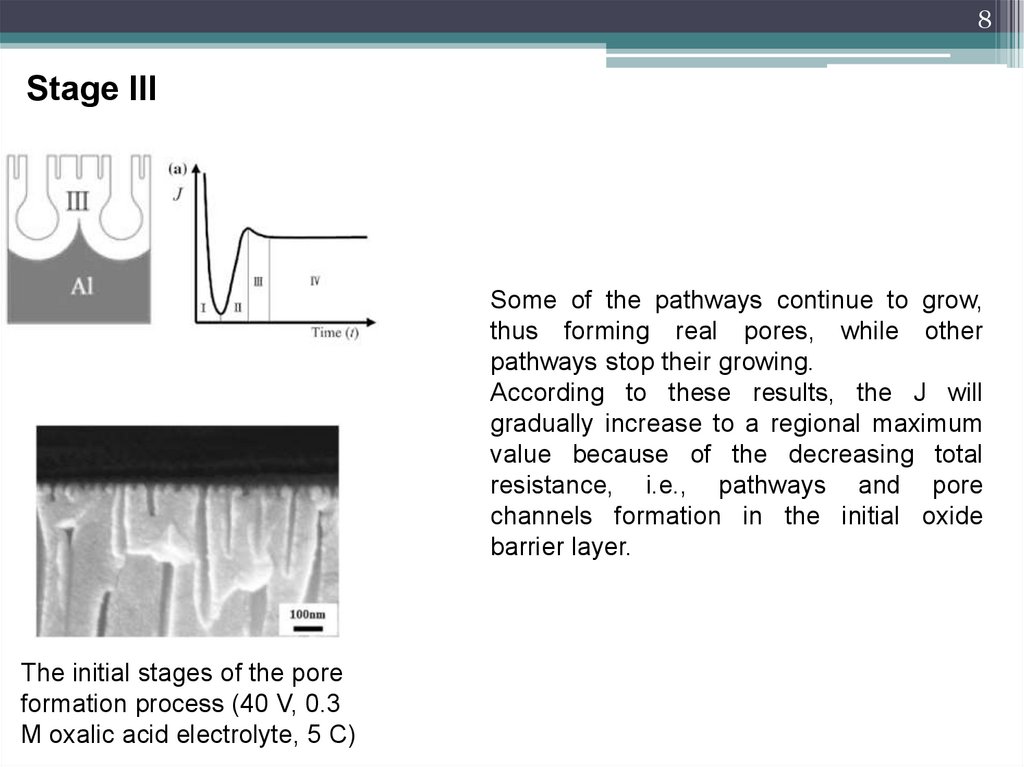
8Stage III
Some of the pathways continue to grow,
thus forming real pores, while other
pathways stop their growing.
According to these results, the J will
gradually increase to a regional maximum
value because of the decreasing total
resistance, i.e., pathways and pore
channels formation in the initial oxide
barrier layer.
The initial stages of the pore
formation process (40 V, 0.3
M oxalic acid electrolyte, 5 C)
9.
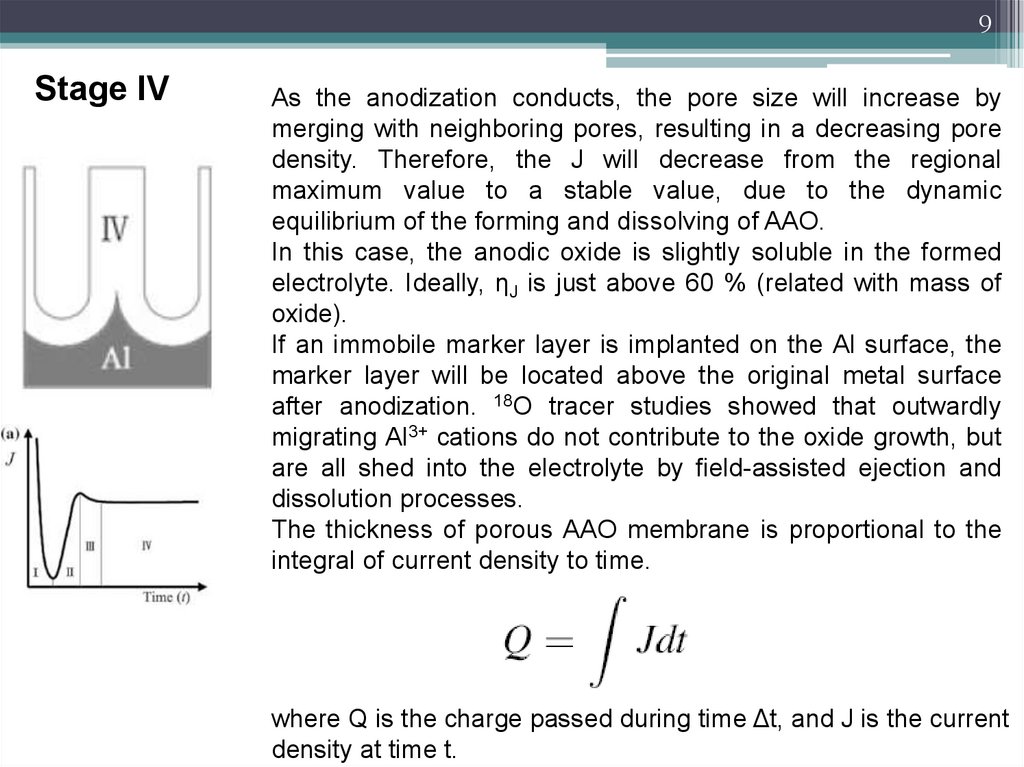
9Stage IV
As the anodization conducts, the pore size will increase by
merging with neighboring pores, resulting in a decreasing pore
density. Therefore, the J will decrease from the regional
maximum value to a stable value, due to the dynamic
equilibrium of the forming and dissolving of AAO.
In this case, the anodic oxide is slightly soluble in the formed
electrolyte. Ideally, ηJ is just above 60 % (related with mass of
oxide).
If an immobile marker layer is implanted on the Al surface, the
marker layer will be located above the original metal surface
after anodization. 18O tracer studies showed that outwardly
migrating Al3+ cations do not contribute to the oxide growth, but
are all shed into the electrolyte by field-assisted ejection and
dissolution processes.
The thickness of porous AAO membrane is proportional to the
integral of current density to time.
where Q is the charge passed during time Δt, and J is the current
density at time t.
10.
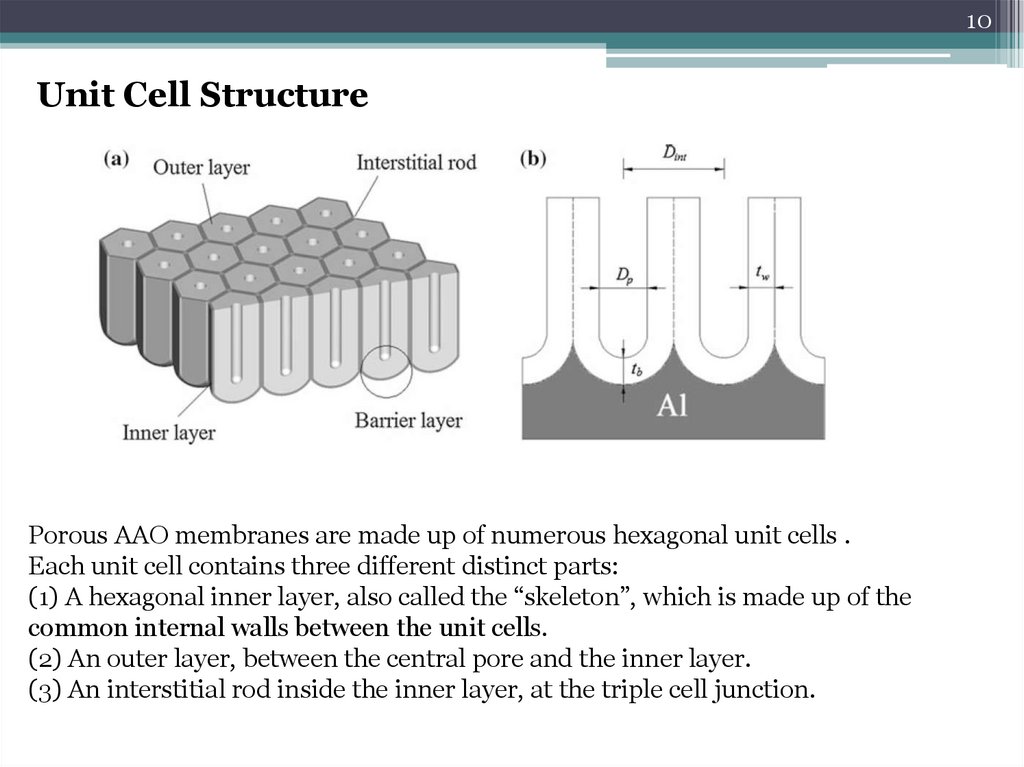
10Unit Cell Structure
Porous AAO membranes are made up of numerous hexagonal unit cells .
Each unit cell contains three different distinct parts:
(1) A hexagonal inner layer, also called the “skeleton”, which is made up of the
common internal walls between the unit cells.
(2) An outer layer, between the central pore and the inner layer.
(3) An interstitial rod inside the inner layer, at the triple cell junction.
11.
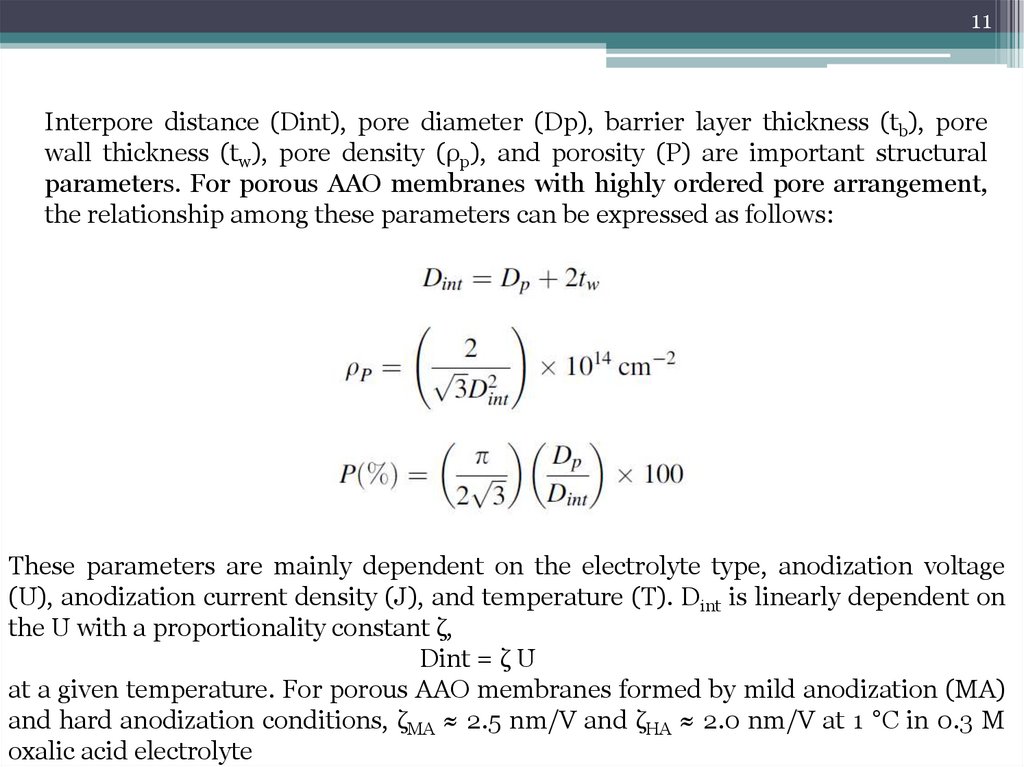
11Interpore distance (Dint), pore diameter (Dp), barrier layer thickness (tb), pore
wall thickness (tw), pore density (ρp), and porosity (P) are important structural
parameters. For porous AAO membranes with highly ordered pore arrangement,
the relationship among these parameters can be expressed as follows:
These parameters are mainly dependent on the electrolyte type, anodization voltage
(U), anodization current density (J), and temperature (T). Dint is linearly dependent on
the U with a proportionality constant ζ,
Dint = ζ U
at a given temperature. For porous AAO membranes formed by mild anodization (MA)
and hard anodization conditions, ζMA ≈ 2.5 nm/V and ζHA ≈ 2.0 nm/V at 1 °C in 0.3 M
oxalic acid electrolyte
12.
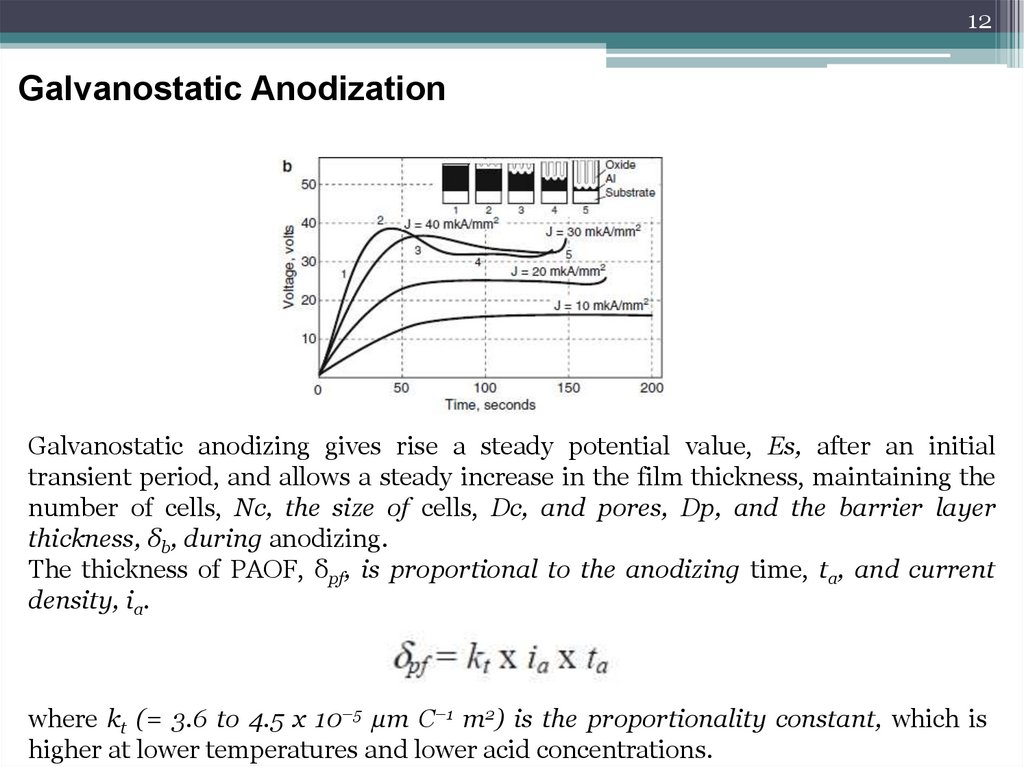
12Galvanostatic Anodization
Galvanostatic anodizing gives rise a steady potential value, Es, after an initial
transient period, and allows a steady increase in the film thickness, maintaining the
number of cells, Nc, the size of cells, Dc, and pores, Dp, and the barrier layer
thickness, δb, during anodizing.
The thickness of PAOF, δpf, is proportional to the anodizing time, ta, and current
density, ia.
where kt (= 3.6 to 4.5 x 10–5 μm C–1 m2) is the proportionality constant, which is
higher at lower temperatures and lower acid concentrations.
13.
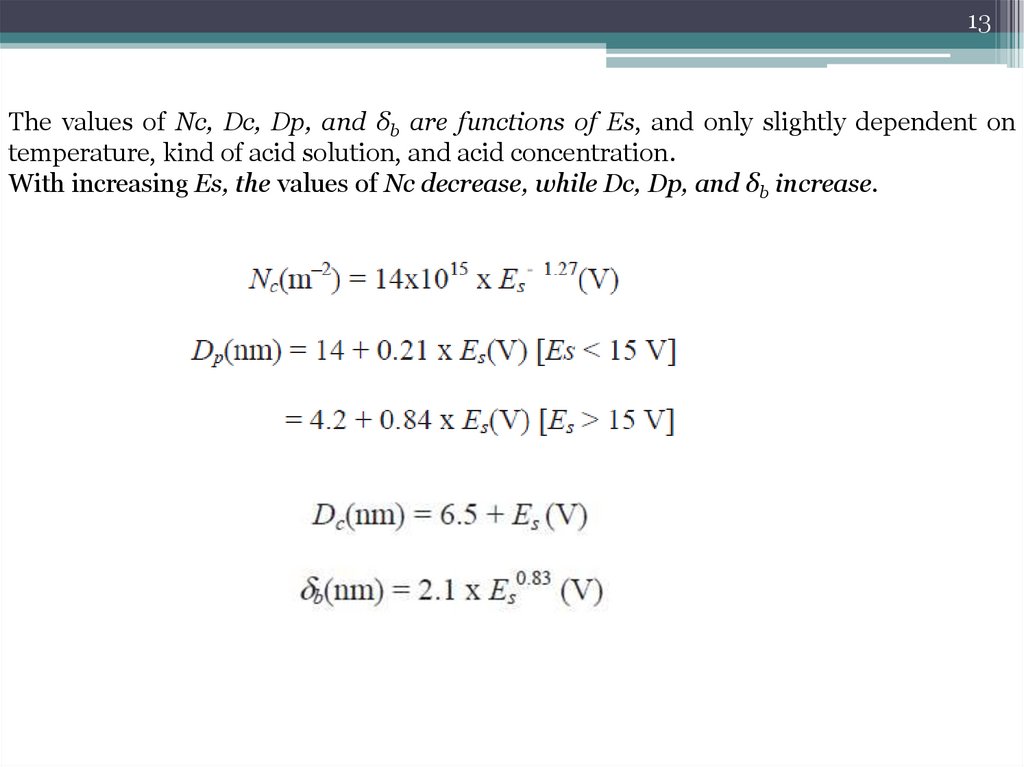
13The values of Nc, Dc, Dp, and δb are functions of Es, and only slightly dependent on
temperature, kind of acid solution, and acid concentration.
With increasing Es, the values of Nc decrease, while Dc, Dp, and δb increase.
14.
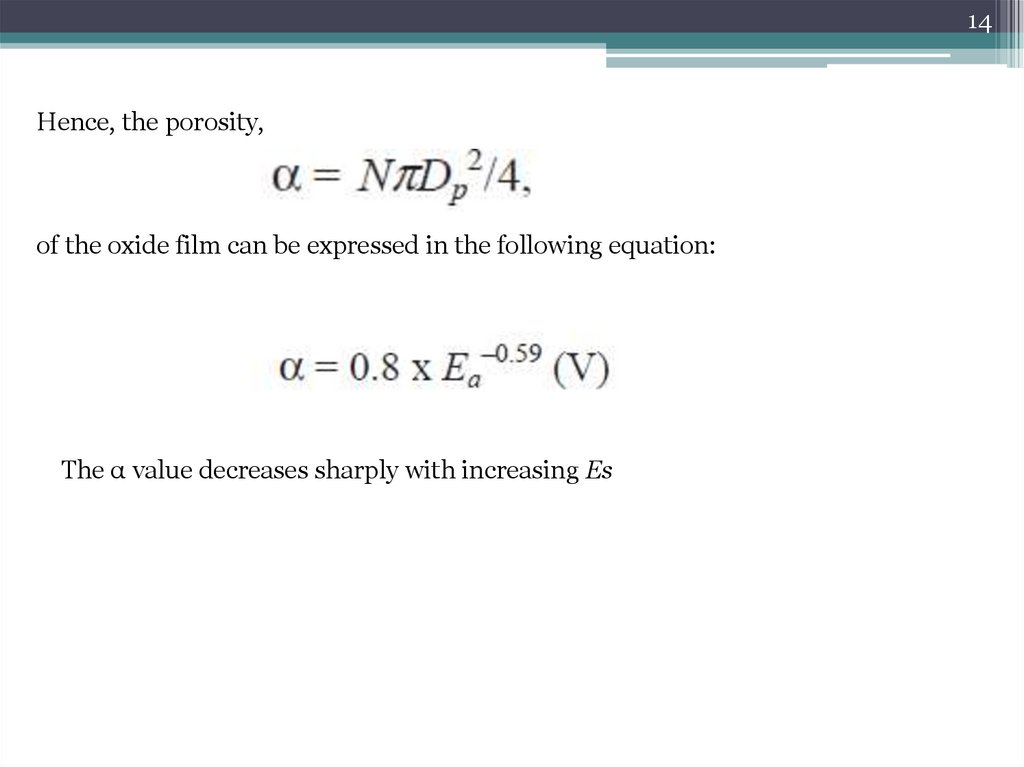
14Hence, the porosity,
of the oxide film can be expressed in the following equation:
The α value decreases sharply with increasing Es


 english
english








