Similar presentations:
Ranscutol® CG
1.
TRANSCUTOL® CGSuperior solubilizer & Efficacy booster
2.
ApplicationsIdentity
Formulation &
Regulation
Transcutol® CG
Process
Ethoxydiglycol
Clinical
evaluation
Mechanism
of action
3.
ApplicationsIdentity
Formulation &
Regulation
Transcutol® CG
Process
Ethoxydiglycol
Clinical
evaluation
Mechanism
of action
4.
IdentityTranscutol® CG
Properties
Efficacy booster
Powerful and versatile solubilizer
Odorless solvent for perfume
extraction
Co-surfactant for microemulsions
Multi-functional
Characteristics
High purity (> 99.5%)
INCI: Ethoxydiglycol
Excellent tolerance profile
5.
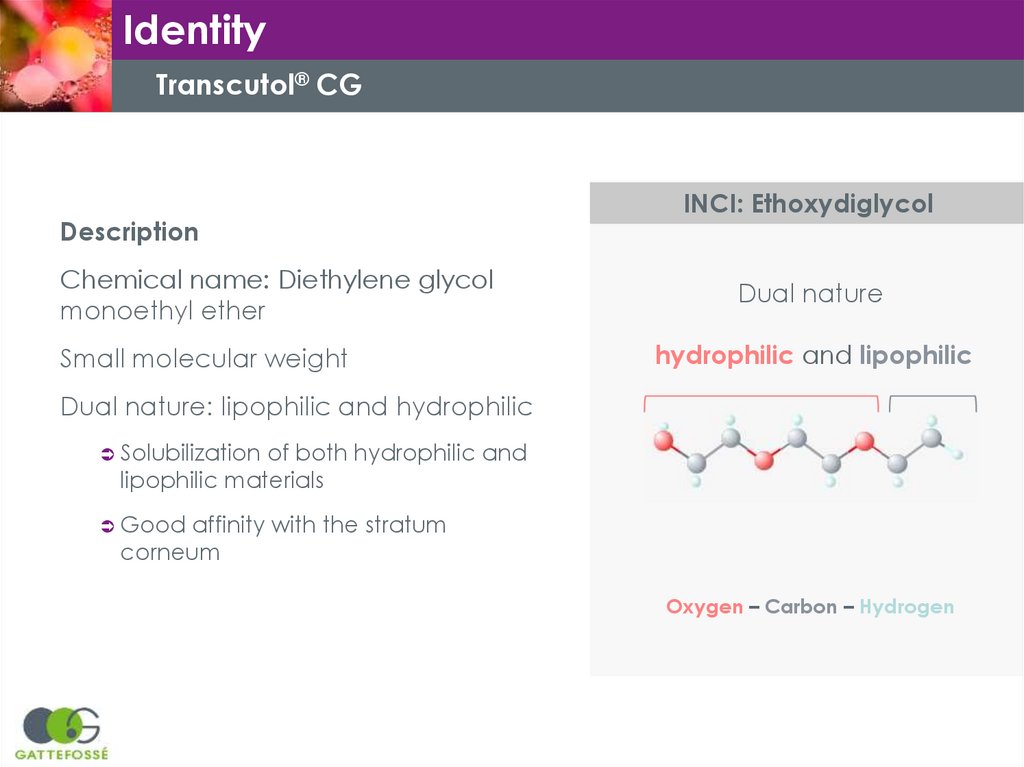
IdentityTranscutol® CG
Description
Chemical name: Diethylene glycol
monoethyl ether
Small molecular weight
INCI: Ethoxydiglycol
Dual nature
hydrophilic and lipophilic
Dual nature: lipophilic and hydrophilic
Solubilization
of both hydrophilic and
lipophilic materials
Good
affinity with the stratum
corneum
Oxygen – Carbon – Hydrogen
6.
ApplicationsIdentity
Formulation &
Regulation
Transcutol® CG
Process
Ethoxydiglycol
Clinical
evaluation
Mechanism
of action
7.
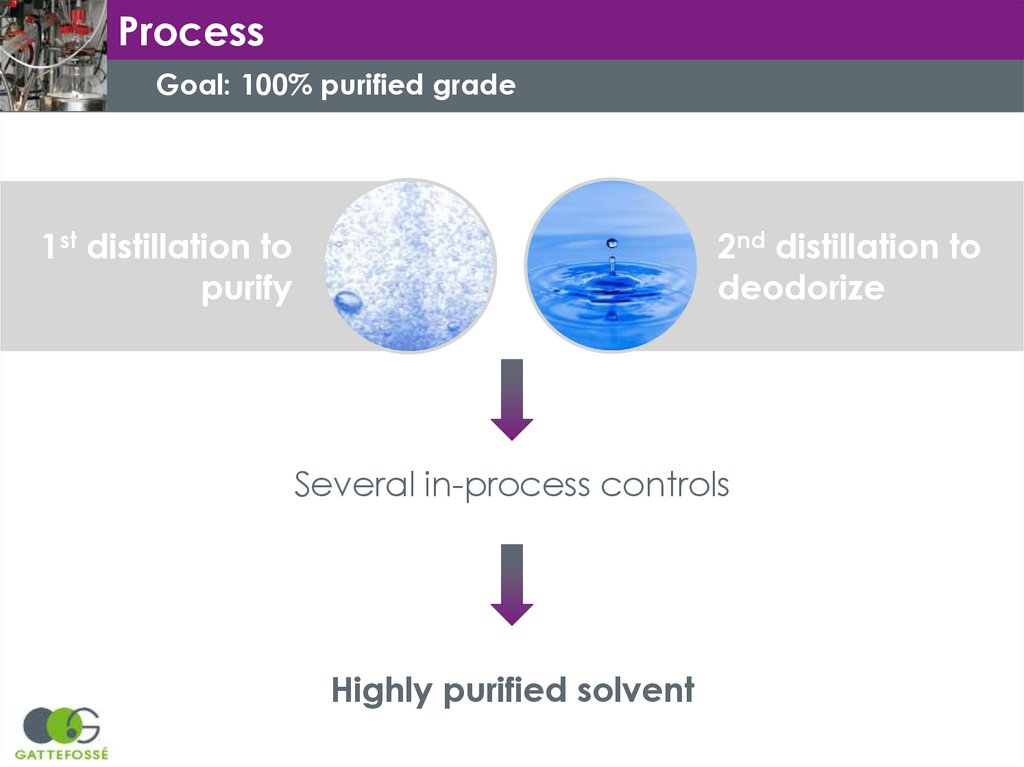
ProcessGoal: 100% purified grade
1st distillation to
purify
2nd distillation to
deodorize
Several in-process controls
Highly purified solvent
8.
ProcessGoal: 100% activity
Purity > 99.5%
9.
ApplicationsIdentity
Formulation &
Regulation
Transcutol® CG
Process
Ethoxydiglycol
Clinical
evaluation
Mechanism
of action
10.
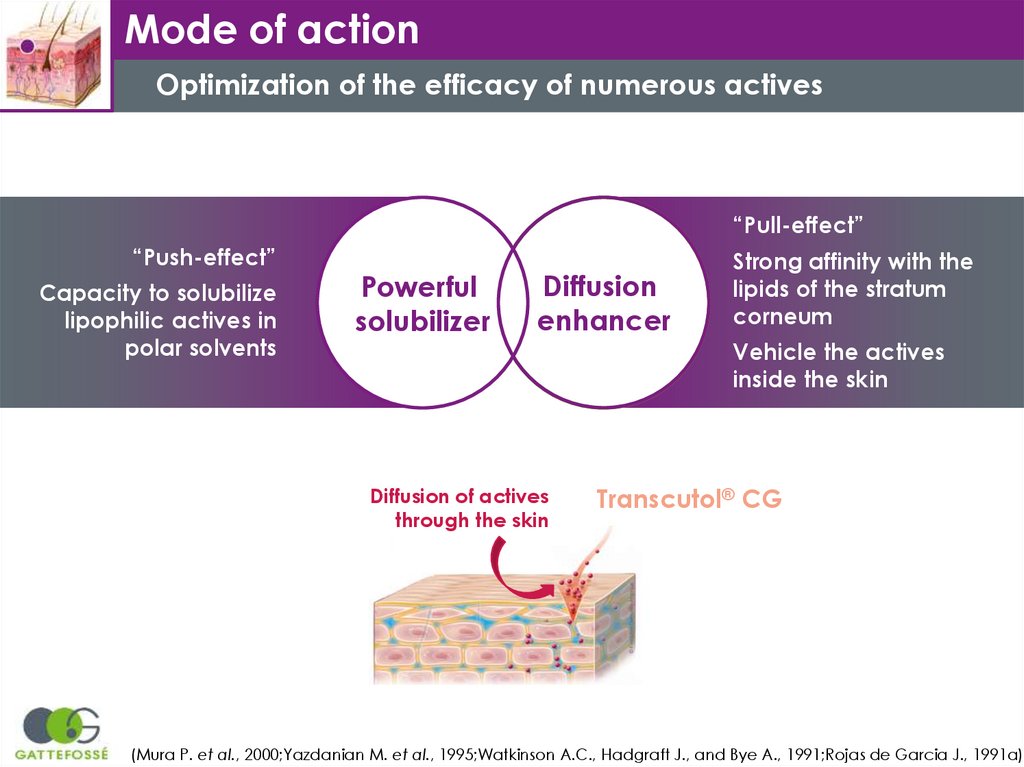
Mode of actionOptimization of the efficacy of numerous actives
“Pull-effect”
“Push-effect”
Capacity to solubilize
lipophilic actives in
polar solvents
Powerful
solubilizer
Diffusion
enhancer
Strong affinity with the
lipids of the stratum
corneum
Vehicle the actives
inside the skin
Diffusion of actives
through the skin
Transcutol® CG
(Mura P. et al., 2000;Yazdanian M. et al., 1995;Watkinson A.C., Hadgraft J., and Bye A., 1991;Rojas de Garcia J., 1991a)
11.
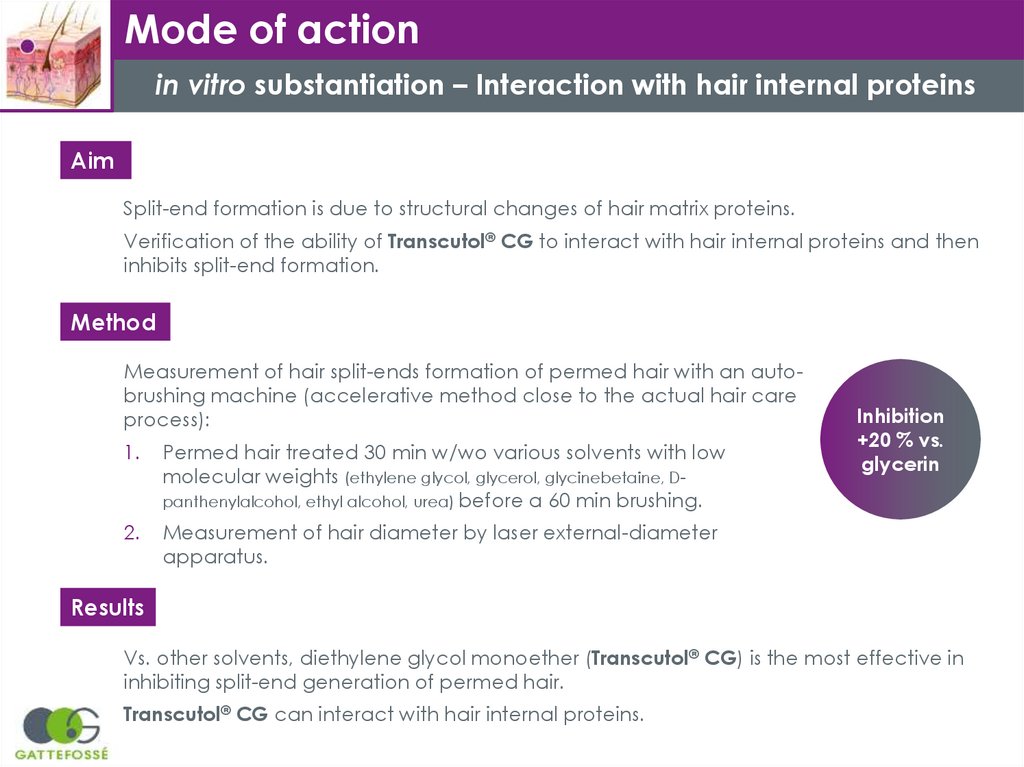
Mode of actionin vitro substantiation – Interaction with hair internal proteins
Aim
Split-end formation is due to structural changes of hair matrix proteins.
Verification of the ability of Transcutol® CG to interact with hair internal proteins and then
inhibits split-end formation.
Method
Measurement of hair split-ends formation of permed hair with an autobrushing machine (accelerative method close to the actual hair care
process):
1.
Permed hair treated 30 min w/wo various solvents with low
molecular weights (ethylene glycol, glycerol, glycinebetaine, Dpanthenylalcohol, ethyl alcohol, urea) before a 60 min brushing.
2.
Measurement of hair diameter by laser external-diameter
apparatus.
Inhibition
+20 % vs.
glycerin
Results
Vs. other solvents, diethylene glycol monoether (Transcutol® CG) is the most effective in
inhibiting split-end generation of permed hair.
Transcutol® CG can interact with hair internal proteins.
12.
ApplicationsIdentity
Formulation &
Regulation
Transcutol® CG
Process
Ethoxydiglycol
Clinical
evaluation
Mechanism
of action
13.
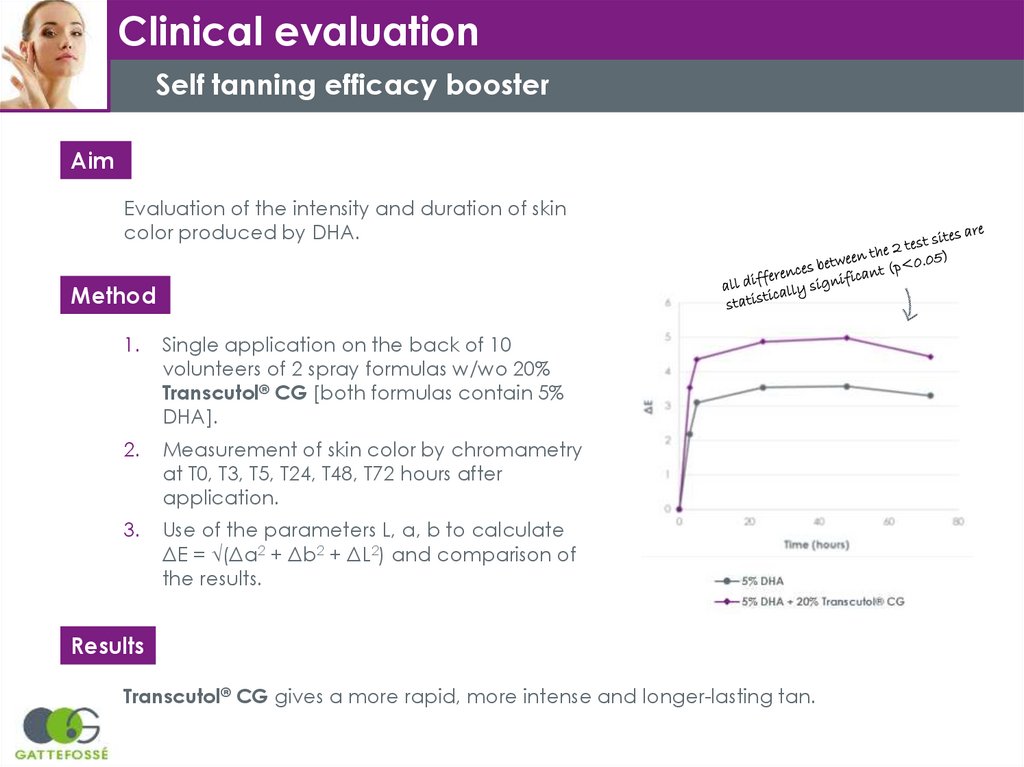
Clinical evaluationSelf tanning efficacy booster
Aim
Evaluation of the intensity and duration of skin
color produced by DHA.
Method
1.
Single application on the back of 10
volunteers of 2 spray formulas w/wo 20%
Transcutol® CG [both formulas contain 5%
DHA].
2.
Measurement of skin color by chromametry
at T0, T3, T5, T24, T48, T72 hours after
application.
3.
Use of the parameters L, a, b to calculate
ΔE = √(Δa2 + Δb2 + ΔL2) and comparison of
the results.
Results
Transcutol® CG gives a more rapid, more intense and longer-lasting tan.
14.
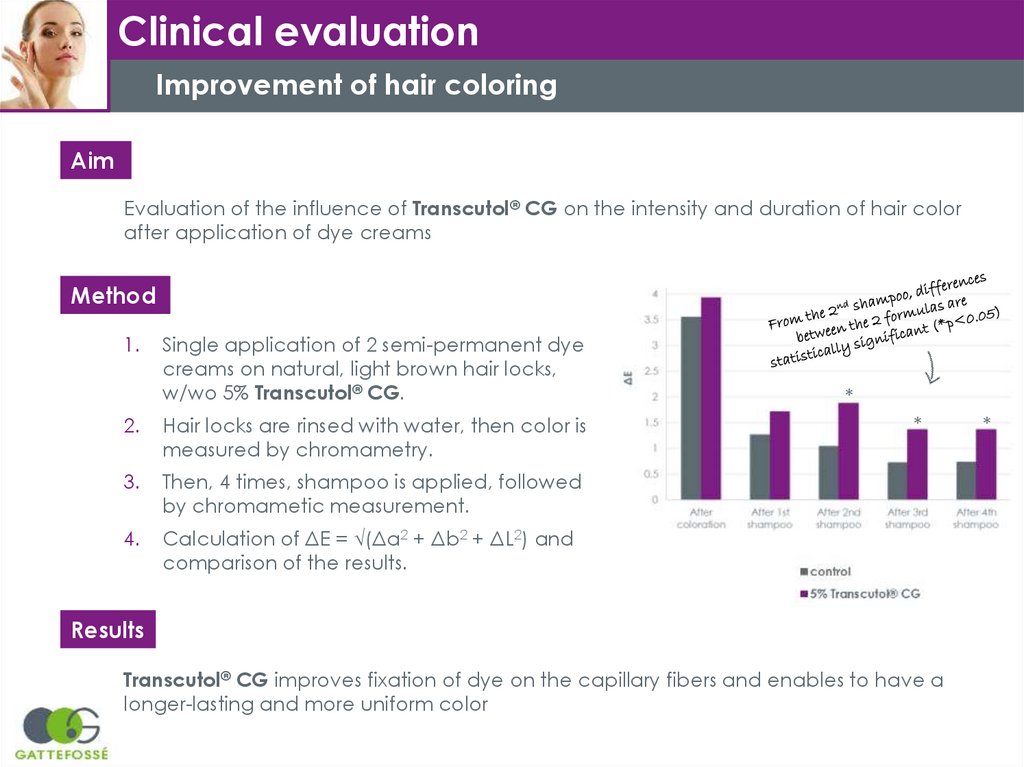
Clinical evaluationImprovement of hair coloring
Aim
Evaluation of the influence of Transcutol® CG on the intensity and duration of hair color
after application of dye creams
Method
1.
Single application of 2 semi-permanent dye
creams on natural, light brown hair locks,
w/wo 5% Transcutol® CG.
2.
Hair locks are rinsed with water, then color is
measured by chromametry.
3.
Then, 4 times, shampoo is applied, followed
by chromametic measurement.
4.
Calculation of ΔE = √(Δa2 + Δb2 + ΔL2) and
comparison of the results.
*
*
Results
Transcutol® CG improves fixation of dye on the capillary fibers and enables to have a
longer-lasting and more uniform color
*
15.
ApplicationsIdentity
Formulation &
Regulation
Transcutol® CG
Process
Ethoxydiglycol
Clinical
evaluation
Mechanism
of action
16.
Formulation & RegulationSafety
Transcutol® has been used in
the pharmaceutical industry for
over 40 years and has an
excellent safety profile
Ocular irritation (single application of 0.1 ml of pure product): non-irritant (score = 9.00).
Acute oral toxicity (single oral administration of the pure product at a dose level of 5,000
mg/kg): non-toxic (LD50 > 5,000 mg/kg).
Primary cutaneous irritation on humans (single application of pure product under occlusive
patch for 48 hours): non-irritant.
Cutaneous sensitization on humans (9 successive applications of pure product under
occlusive patch for 48 hours over a 3-week period 14 days of rest—challenge application):
non-irritant and non-sensitizing.
Dermal toxicity by repeated applications for 28 days (daily application under patch for 6
hours/day for a 28-day period, at levels of 100, 300 and 1,000 mg/kg/day): non-toxic.
17.
Formulation & RegulationFormulation
o
o
Recommended use level: 2-30%*
Soluble in water
Soluble in alcohol
Insoluble in oils
Process:
o
Slowly add the active to the
Transcutol® CG
Mix until the active is completely
dissolve
Add Transcutol® CG at a temperature < 50°C
(risk of oxidation above)
*see restricted use levels in Europe
18.
Formulation & RegulationRegulatory status
o
o
Worldwide regulations
Worldwide approval (restricted use levels in Europe)
China: listed IECIC 2014-06
Australia TGA
Japan QD
Restricted use levels in Europe:
Rinse-off products: up to 10%
Oxidative hair dye formulations: up to 7%
Non-oxidative hair dye formulations: up to 5%
Spray products, fine fragrances, hair sprays,
antiperspirants/deodorants: up to 2.6%
All cosmetic products: up to 2.6%
19.
ApplicationsIdentity
Formulation &
Regulation
Transcutol® CG
Process
Ethoxydiglycol
Clinical
evaluation
Mechanism
of action
20.
ApplicationsSunscreens
Optimized UVA protection
Commonly used sun filter: Benzophenone-3
Crystalline powder with a tendency to crystallize
out of many formulations, especially when < 3%
in low oil content formulations
Transcutol® CG minimizes crystallization and
increases efficacy
Optimized UVB protection
Commonly used sun filter: Octyl Methoxycinnamate (OMC)
In non-polar solvents, the UV curve of OMC will shift to a shorter wavelength
With Transcutol® CG, the UV curve of OMC remains centered at 308 nm in
sunscreen formulations with a low polarity oil vehicle, Transcutol® CG will increase
the polarity of the medium
Sensoriality
Transcutol® CG helps to reduce the oiliness of the sunscreens
21.
ApplicationsToiletries
Antiperspirants
Transcutol® CG improves skin feel and decreases
tacky feel
Transcutol® CG facilitates aluminum
chlorohydrate dispersion and then increases
efficacy
Soaps
Transcutol® CG decreases the dissolution time of Triclocarban, antimicrobial active
Transcutol® CG improves its uniform distribution throughout the soap
22.
ApplicationsOthers
o
Odorless solvent for perfume
extraction
Superior solubilizing properties
Olfactive neutrality
Low irritation potential
o
Anti-acne preparations
o
Solubilization of essences and aromatic
compounds
Solubilization of Benzoyl peroxide to avoid
grinding of this potentially explosive oxidizing
agent, and avoid grainy and scratching
sensation
Co-surfactant for microemulsions
23.
ApplicationsTranscutol® CG
Applications
Compatible
with all types of formulations
Specific applications
Skin Care:
Anti-acne
preparations
Sun Care:
Sunscreens
Self-tanning
products
Hair care:
Conditioners
Hair
dyes
Toiletries:
Antiperspirants
Antimicrobial
soaps
24.
Transcutol® CGINCI: Ethoxydiglycol
Key features:
o
Efficacy booster
o
Powerful and versatile solubilizer
o
High purity (> 99.5%)
o
Excellent tolerance profile
o
Compatible with all types of
formulations








 medicine
medicine advertising
advertising








