Similar presentations:
Selenium
1. Selenium
2.
3. History
Selenium (Greek σελήνη selene meaning "Moon") wasdiscovered in 1817 by Jöns Jacob Berzelius, who noted
the similarity of the new element to the previously
discovered tellurium (named for the Earth).
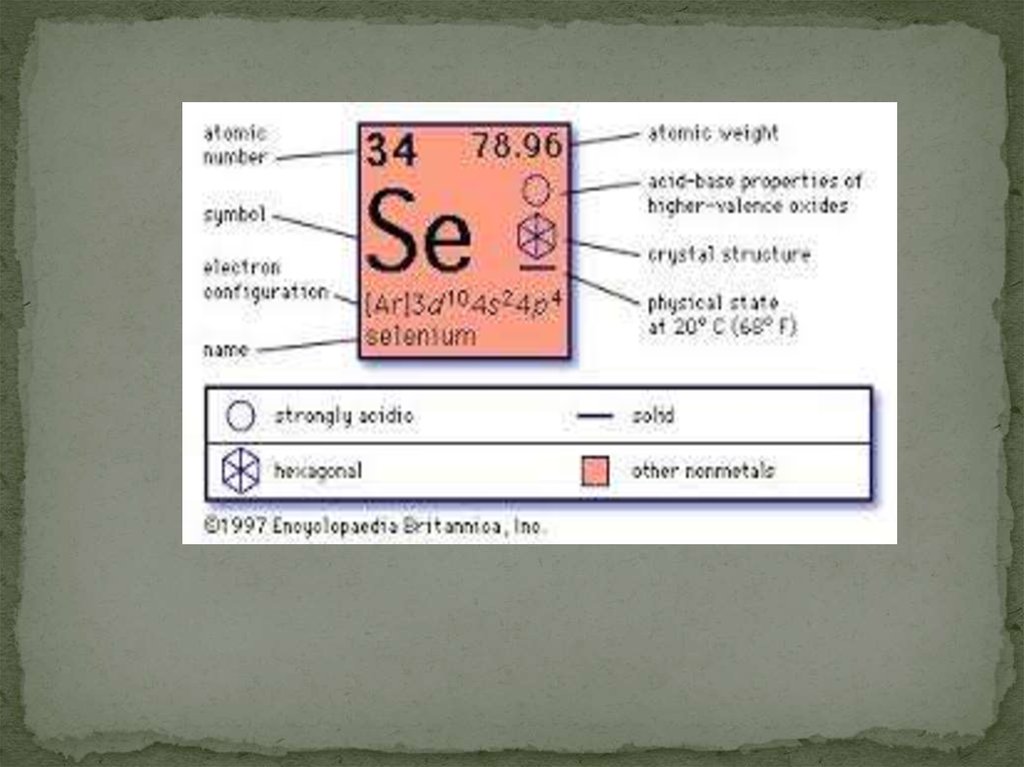
4. Physical properties
black, red, and gray (not pictured) allotropesSelenium salts are toxic in large amounts
Nonmetal
Hard
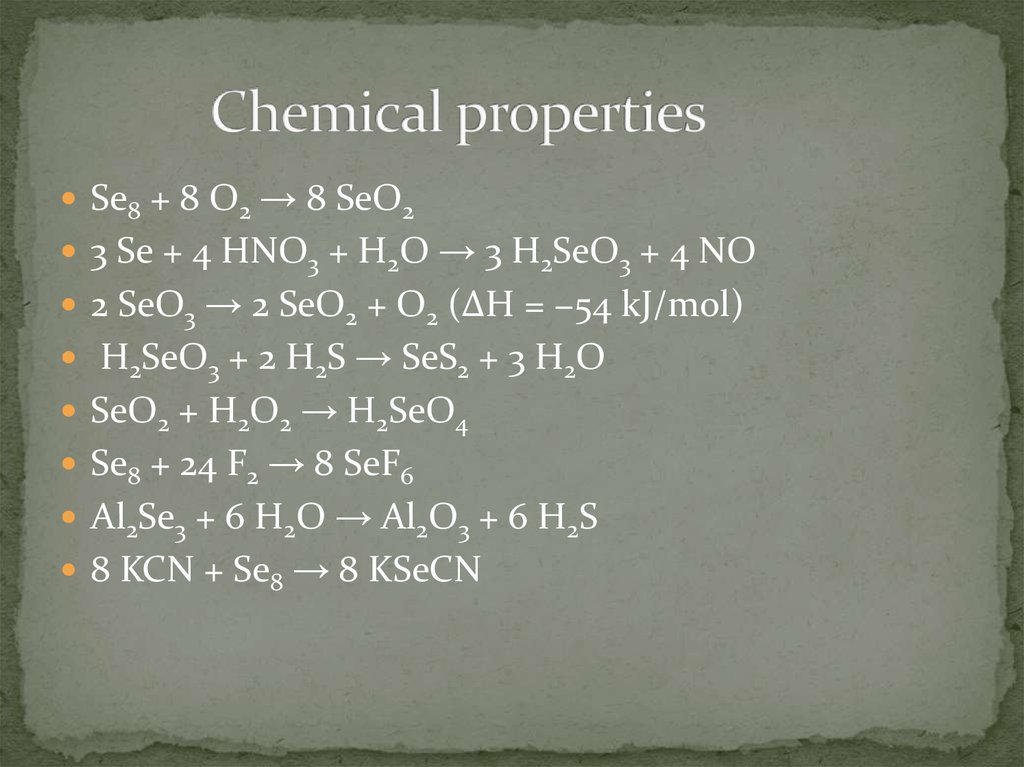
5. Chemical properties
Se8 + 8 O2 → 8 SeO23 Se + 4 HNO3 + H2O → 3 H2SeO3 + 4 NO
2 SeO3 → 2 SeO2 + O2 (ΔH = −54 kJ/mol)
H2SeO3 + 2 H2S → SeS2 + 3 H2O
SeO2 + H2O2 → H2SeO4
Se8 + 24 F2 → 8 SeF6
Al2Se3 + 6 H2O → Al2O3 + 6 H2S
8 KCN + Se8 → 8 KSeCN
6. Compounds
H2SeO3selenous acid2 SeO3 trioxide
(K2SeO4) anhydrous potassium selenate
(Ag2SeO3) silver selenite
(Na2SeO3) sodium selenite
SeS2 Selenium disulfide
H2SeO4 hydrogen peroxide
(SeOCl2) selenium oxychloride
7.
8. Distribution in nature.
Selenium is a rare and dispersed element; its content in theearth’s crust (clarke) is 5 X 10–6 percent by weight. The
history of selenium in the earth’s crust is closely linked to
that of sulfur. Selenium displays a tendency toward
concentration and, despite its low clarke, forms 38
minerals of its own, including selenides, selenites, and
selenates. Isomor-phous admixtures of selenium are
characteristic in sulfides and native sulfur.
Selenium undergoes active migration in the biosphere.
Igneous rocks, volcanic gases, and volcanic hot springs are
sources of selenium accumulations in organisms. For this
reason, the soils and sedimentary rocks in active and
dormant volcanic regions are often enriched with
selenium. Here, the average content in clays and shales is 6
× 10–5 percent.
9. Uses
In the vulcanizationCatalyst in some chemical reactions
Toning of photographic prints
Se is used as a gamma source in industrial radiography
10.
11. Medical use
The substance loosely called seleniumsulfide (approximate formula, SeS2) is the active
ingredient in some anti-dandruff shampoos. The
selenium compound kills the scalp fungus Malassezia,
which causes shedding of dry skin fragments. The
ingredient is also used in body lotions to treat tinea
versicolor due to infection by a different species
of Malassezia fungus.
12.
13. In Kazakhstan
We can find selenium and Tellurium in Altai, vicinityof the mountains Chingiz and Sayan,
Konyrat,Shatyrkol , Zhezkazgan
14. By:
Ratbek YryszhanBukharbayeva Makpal
Kaldybekova Zilola
Ramisova Ruzhdie
Asan Tansholpan












 chemistry
chemistry








