Similar presentations:
Separation amp confirmation
1.
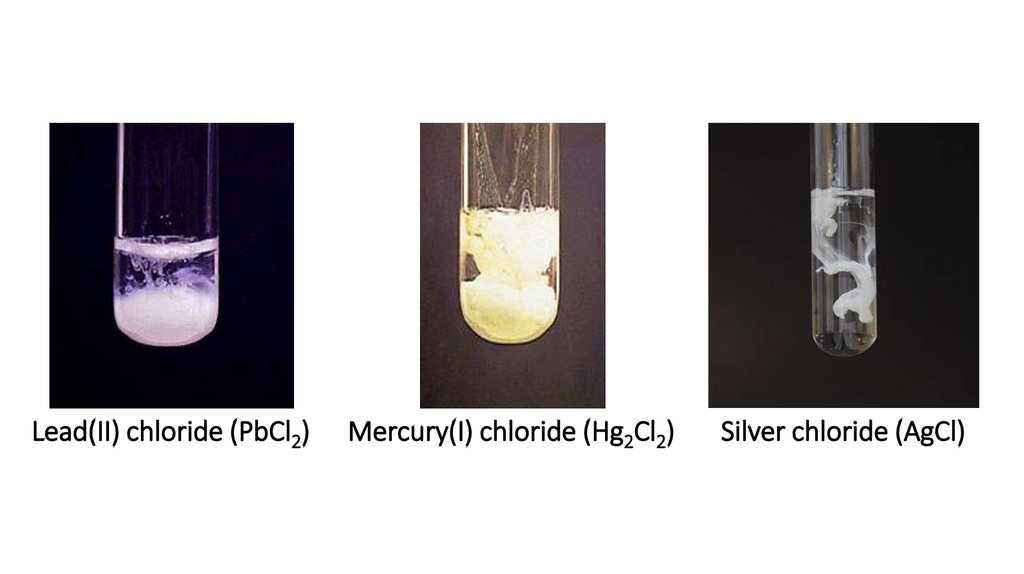
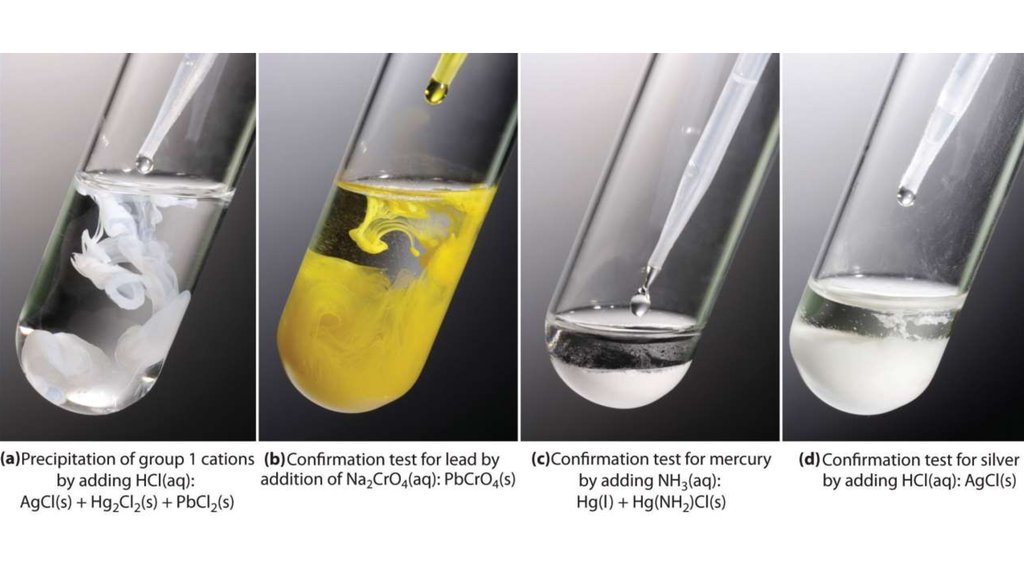
Lead(II) chloride (PbCl2)Mercury(I) chloride (Hg2Cl2)
Silver chloride (AgCl)
2.
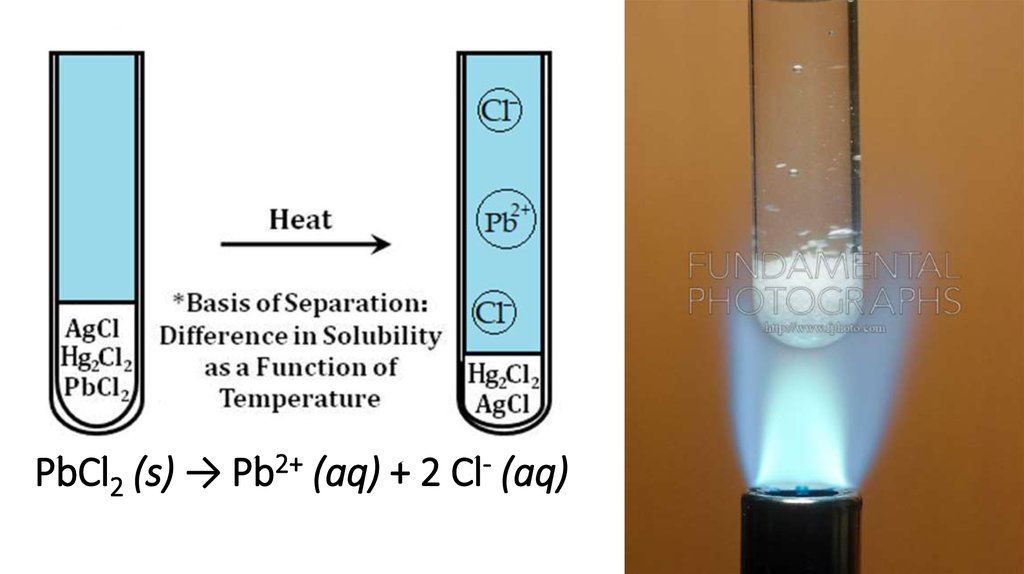
PbCl2 (s) → Pb2+ (aq) + 2 Cl- (aq)3.
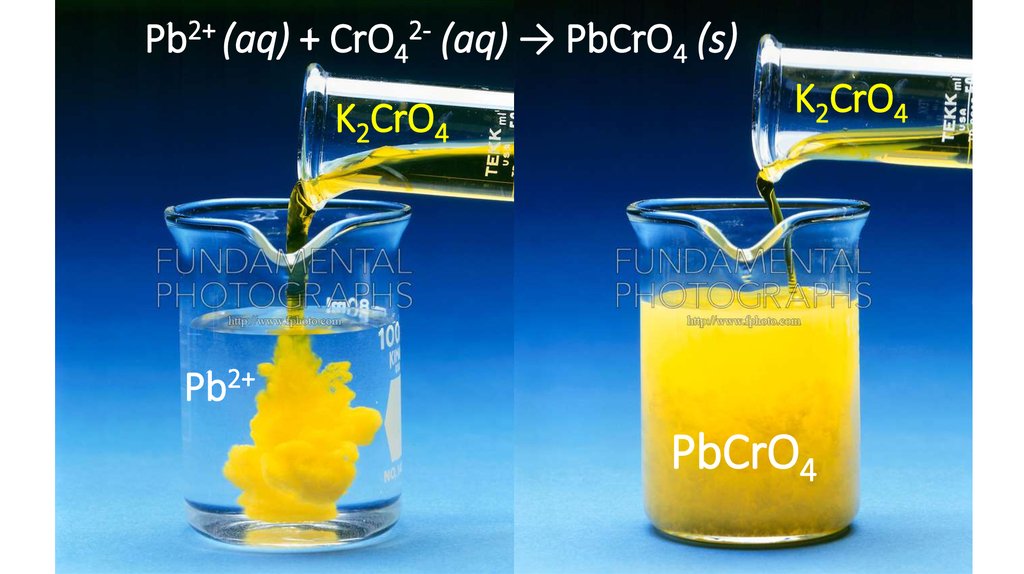
Pb2+ (aq) + CrO42- (aq) → PbCrO4 (s)K2CrO4
K2CrO4
Pb2+
PbCrO4
4.
5.
AgCl (s) + 2 NH3 (aq) → Ag(NH3)2+ (aq) + Cl- (aq)NH3
AgCl
NH3
NH3
Ag(NH3)2Cl
NH3
6.
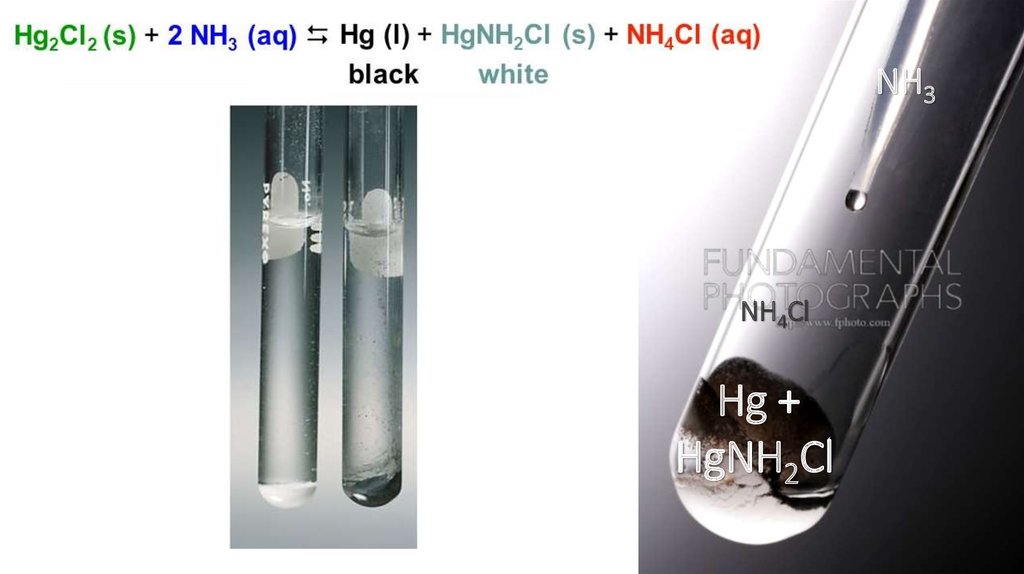
NH3NH4Cl
Hg +
HgNH2Cl
7.
Ag(NH3)2+ (aq) + 2 H+ (aq) → Ag+ (aq) + 2 NH4+ (aq)Ag+ (aq) + Cl- (aq) → AgCl (s)
HNO3
HNO3
NH4NO3
NH4NO3
Ag(NH3)2Cl
AgCl
AgCl
HNO3




 chemistry
chemistry








