Similar presentations:
V-HeFT I and V-HeFT II Trials― The Path to A-HeFT
1. V-HeFT I and V-HeFT II Trials― The Path to A-HeFT
CV-126
V-HeFT I and V-HeFT II Trials―
The Path to A-HeFT
Jay N. Cohn, MD
Professor of Medicine
University of Minnesota Medical School
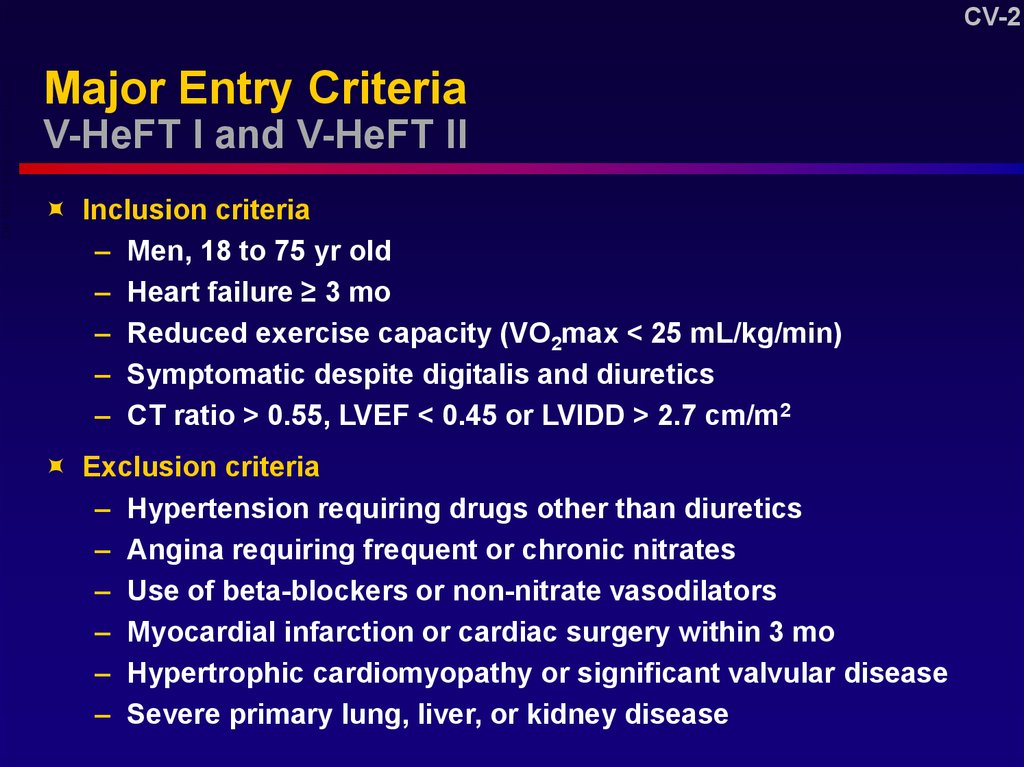
2. Major Entry Criteria V-HeFT I and V-HeFT II
CV-2DV BiDil FDA slides10.ppt
26
Major Entry Criteria
V-HeFT I and V-HeFT II
Inclusion criteria
– Men, 18 to 75 yr old
– Heart failure ≥ 3 mo
– Reduced exercise capacity (VO2max < 25 mL/kg/min)
– Symptomatic despite digitalis and diuretics
– CT ratio > 0.55, LVEF < 0.45 or LVIDD > 2.7 cm/m2
Exclusion criteria
– Hypertension requiring drugs other than diuretics
– Angina requiring frequent or chronic nitrates
– Use of beta-blockers or non-nitrate vasodilators
– Myocardial infarction or cardiac surgery within 3 mo
– Hypertrophic cardiomyopathy or significant valvular disease
– Severe primary lung, liver, or kidney disease
3. Study Plan V-HeFT I and V-HeFT II
CV-3DV BiDil FDA slides10.ppt
26
Study Plan
V-HeFT I and V-HeFT II
V-HeFT I
Placebo
n = 276
Prazosin 5 mg qid
n = 183
2.3 yr
(0.5 - 5.7 yr)
Hydralazine 75 mg qid n = 186
Isosorbide dinitrate 40 mg qid
Enalapril 10 mg bid
n = 403
V-HeFT II
2.5 yr
(0.5 - 4.9 yr)
Hydralazine 75 mg qid n = 401
Isosorbide dinitrate 40 mg qid
4. Study Endpoints V-HeFT I and V-HeFT II
CV-4DV BiDil FDA slides10.ppt
26
Study Endpoints
V-HeFT I and V-HeFT II
Major endpoints
– All-cause mortality during entire study
– All-cause mortality at 2 yr
– Number and duration of cardiovascular
hospitalizations
– Maximum oxygen consumption at peak exercise
– Quality of life (V-HeFT II)
5. Survival in All Patients V-HeFT I
CV-59
Survival in All Patients
V-HeFT I
ISDN/HYD
Placebo
Prazosin
Survival, %
100
75
50
25
P = 0.093
ISDN/HYD vs placebo
0
365
730
1095
1460
1825
Days since randomization date
ISDN/HYD, n = 186
Placebo, n = 276
Prazosin, n = 183
148
202
135
109
135
94
71
84
58
37
41
27
16
10
7
6. Survival in All Patients V-HeFT I
CV-6DV Final NDA20-727_BD.pdf T 3 and pg 26, 24T3
26
Survival in All Patients
V-HeFT I
V-HeFT I, overall
Treatment
Placebo,
n (%)
Drug,
n (%)
Hazard ratio
(95% CI)
Log-rank
P value
ISDN/HYD
120 (44.0) 72 (38.7)
0.78 (0.58, 1.04)
0.093
Prazosin
120 (44.0) 91 (49.7)
1.11 (0.85, 1.46)
0.441
V-HeFT I, endpoint of 2 yr
Placebo, %
ISDN/HYD, %
P value
34.3
25.6
0.053
7. Survival in All Patients V-HeFT II
CV-7Survival in All Patients
V-HeFT II
100
ISDN/HYD
Enalapril
90
Survival, %
DV Final NDA20-727_Brief_Document.pdf F 8
26
80
70
60
HR = 1.23 (0.97, 1.55)
Log-rank P = 0.083
50
40
0
365
730
1095
1460
1825
Time, days since randomization
ISDN/HYD, n = 401
Enalapril, n = 403
332
346
242
265
157
169
86
89
3
1
8. Survival in All Patients V-HeFT II
CV-8DV Final NDA20-727_Brief_Document.pdf page 46
26
Survival in All Patients
V-HeFT II
V-HeFT II, overall
Enalapril
n = 403
ISDN/HYD
n = 401
Hazard ratio
(95% CI)
Log-rank
P value
132 (32.8%)
153 (38.2%)
1.23 (0.97, 1.55)
0.083
V-HeFT II, endpoint of 2 yr
Enalapril, %
ISDN/HYD, %
P value
18.0
25.0
0.016
9. Subgroup Analysis
CV-9Subgroup Analysis
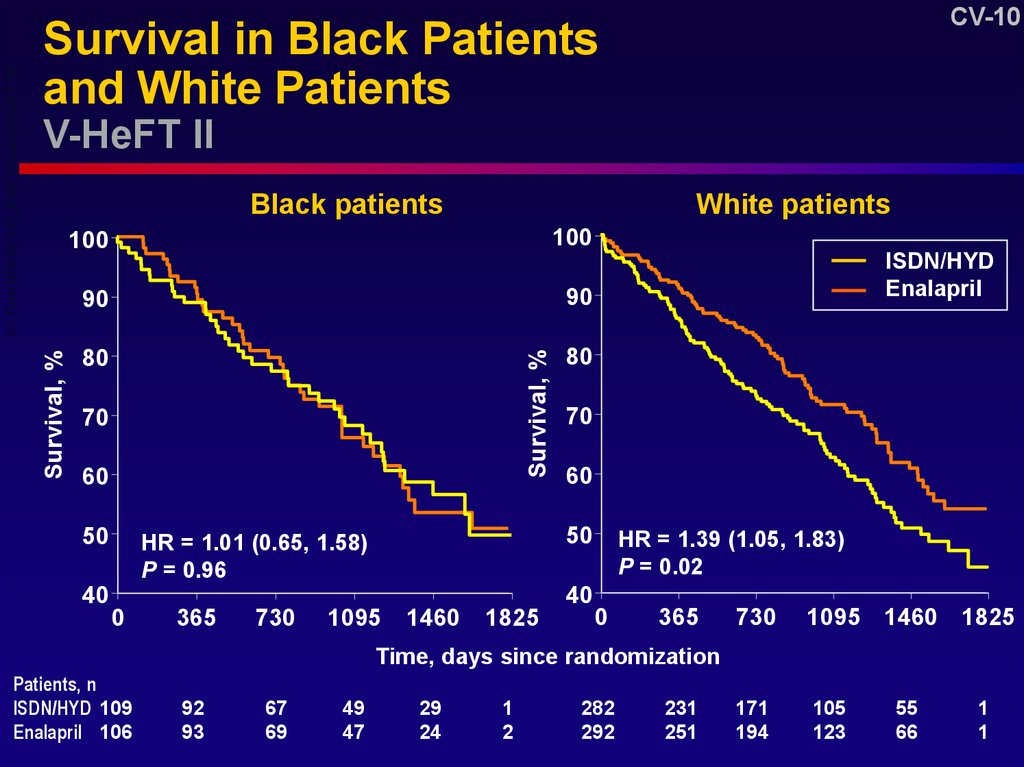
10. Survival in Black Patients and White Patients V-HeFT II
Black patientsWhite patients
100
100
90
90
80
80
Survival, %
Survival, %
DV Final NDA20-727_Brief_Document.pdf F 10
CV-10
70
60
50
70
60
50
HR = 1.01 (0.65, 1.58)
P = 0.96
40
40
0
365
730
ISDN/HYD
Enalapril
1095
1460
1825
HR = 1.39 (1.05, 1.83)
P = 0.02
0
365
730
1095 1460 1825
171
194
105
123
Time, days since randomization
Patients, n
ISDN/HYD 109
Enalapril 106
92
93
67
69
49
47
29
24
1
2
282
292
231
251
55
66
1
1
11. Survival in Black Patients and White Patients V-HeFT I
Black patients100
90
90
80
80
70
60
60
50
40 HR = 0.53 (0.29, 0.98)
40
30
0
30
P = 0.04
730
1095
1460
1825
ISDN/HYD
Placebo
70
50
365
White patients
100
Survival, %
Survival, %
DV Final NDA20-727_Brief_Document.pdf F 3
CV-11
HR = 0.88 (0.63, 1.24)
P = 0.47
0
365
730
1095 1460 1825
Time, days since randomization
Patients, n
ISDN/HYD 49
Placebo 79
43
61
36
44
28
29
16
14
8
3
132
192
102
140
71
91
42
55
22
27
9
8
12. V-HeFT I—Conclusions (1)
CV-12V-HeFT I—Conclusions (1)
ISDN/HYD compared to placebo was
associated with
– A 22% lower risk of death overall (P = 0.09)
– A 12% lower risk of death in white patients
(P = 0.47)
– A 47% lower risk of death in black patients
(P = 0.04)
13. V-HeFT II—Conclusions (2)
CV-13V-HeFT II—Conclusions (2)
Enalapril compared to ISDN/HYD was
associated with
– A 23% lower mortality overall (P = 0.08)
– A 39% lower mortality in white patients
(P = 0.02)
– No difference in mortality in blacks
14. From V-HeFT I and V-HeFT II to A-HeFT
CV-14From V-HeFT I and V-HeFT II to A-HeFT
Based on V-HeFT I and V-HeFT II, a clinical
study was needed to confirm the hypothesis
that the ISDN/HYD combination benefits
outcomes in black HF patients
A-HeFT was designed as a prospective,
placebo-controlled study with the objective of
testing BiDil’s effects on survival, heart failure
hospitalizations, and quality of life in patients
receiving contemporary therapy for heart
failure












 medicine
medicine