Similar presentations:
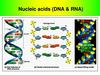
Functions and structures of DNA and nucleotide
1.
What do you know about these scientists?2. Functions and structures of DNA and nucleotide
3. Learning objectives
• 11.4.1.8 establish a link between DNA structureand its function
• 11.4.1.9 describe the chemical structure of the
nucleotides and explain their connection and
location in the DNA molecule
4. success criteria
DNA:1. Knows the structure of DNA.
2. Describes the functions of DNA.
3. Establish a link between DNA structure to its function.
Nucleeotide:
1. Knows the chemical structure of a nucleotide.
2. Describes their connection.
3. Explain how the nucleotides are located in the DNA.
5. Terminology
• DNA/ nucleotide• Purines/pyremidines
• Adenine/guanine/ cytosine/ thymine
• Monomer/polymer
• Phosphate group/pentose sugar/ deoxyribose/ nitrogenous-bases
• H-bond/ covalent bond/ ester bond/ glycoside bond
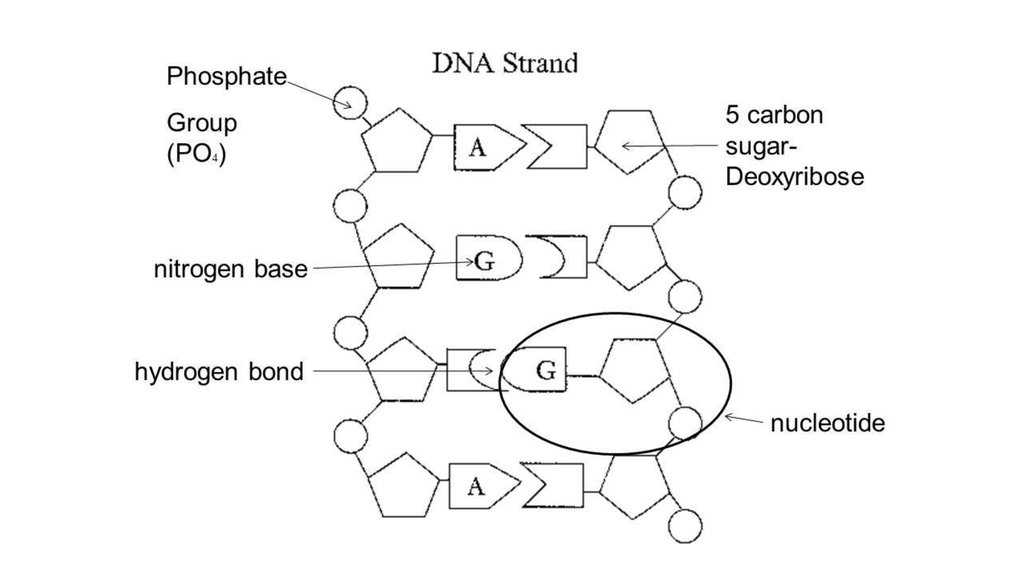
6. DNA
DNA stands for deoxyribonucleic acid. DNA isalso polymer, made up of many similar,
smaller molecules joined into a long chain.
The smaller molecules from which DNA
molecules are made are nucleotides. DNA is
therefore polynucleotides. They are often
referred to simply as nucleic acids.
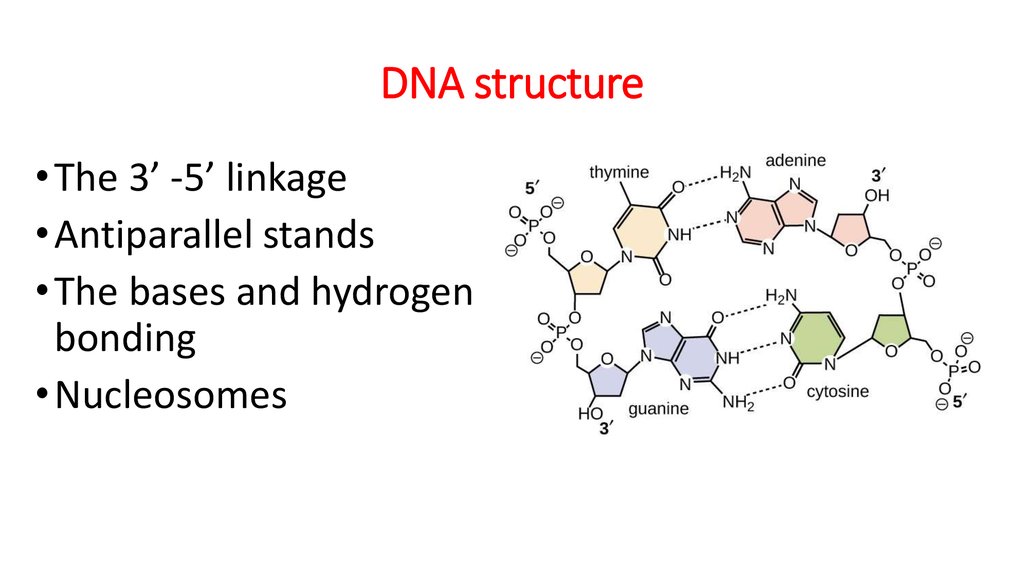
7. DNA structure
• The 3’ -5’ linkage• Antiparallel stands
• The bases and hydrogen
bonding
• Nucleosomes
8. The 3’ -5’ linkage
The carbons in the sugar are numbered from 1 to 5 in a clockwise direction startingafter the oxygen at the apex.
The base is attached to carbons 1.
Carbon 2 has just a hydrogen attached instead of an OH group. (called - deoxyribose)
Carbon 3 is where te next nucleotide attaches in one direction.
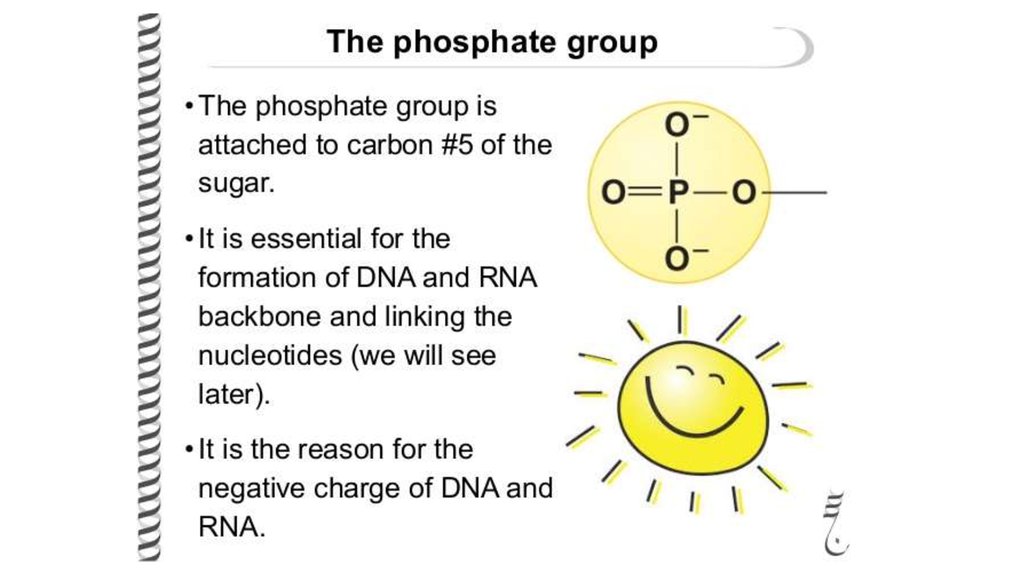
Carbon 5 has a phosphate group attached to it.
This means that each nucleotide is linked to those on
either side of it through carbons 3 and 5.
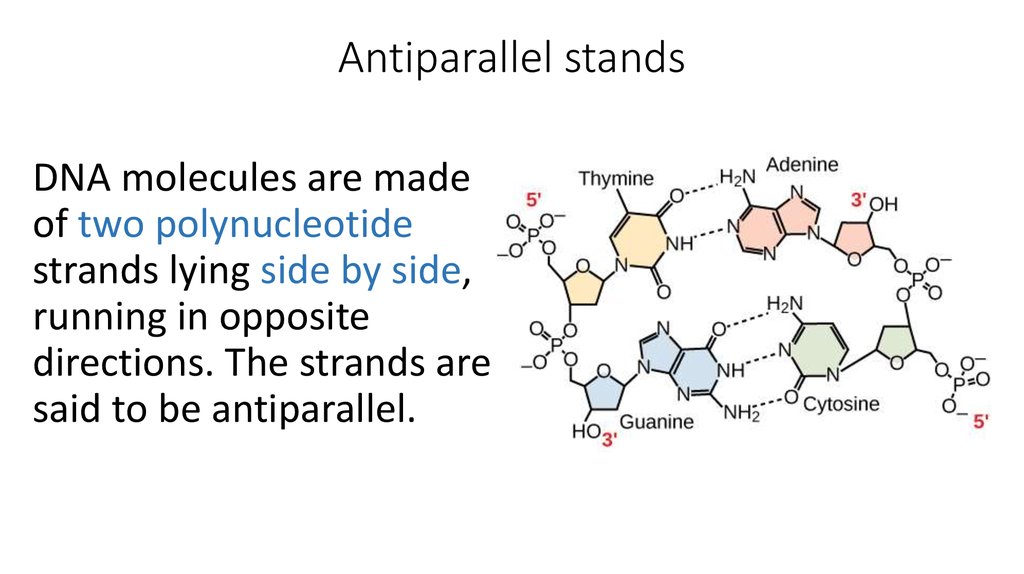
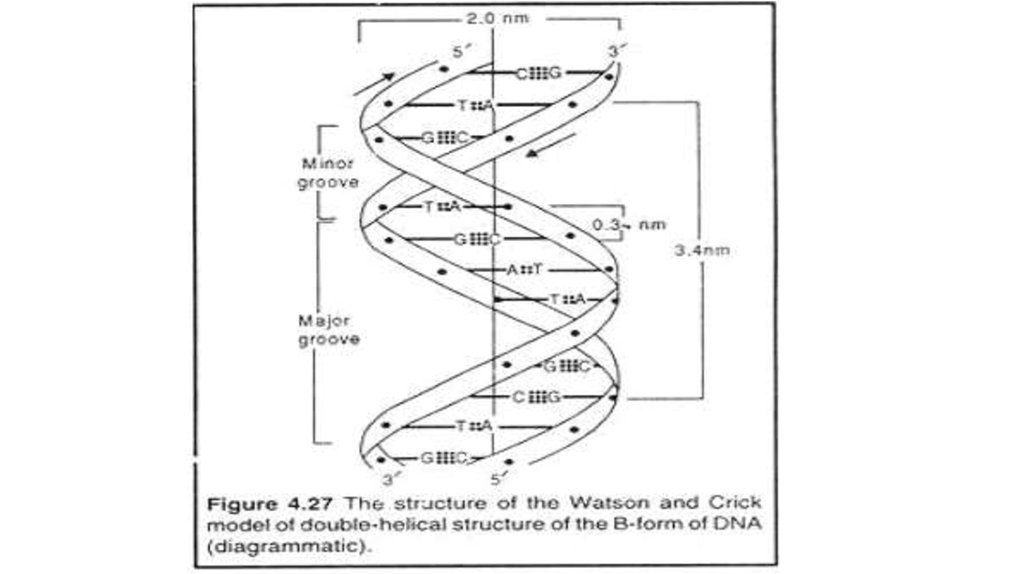
9. Antiparallel stands
DNA molecules are madeof two polynucleotide
strands lying side by side,
running in opposite
directions. The strands are
said to be antiparallel.
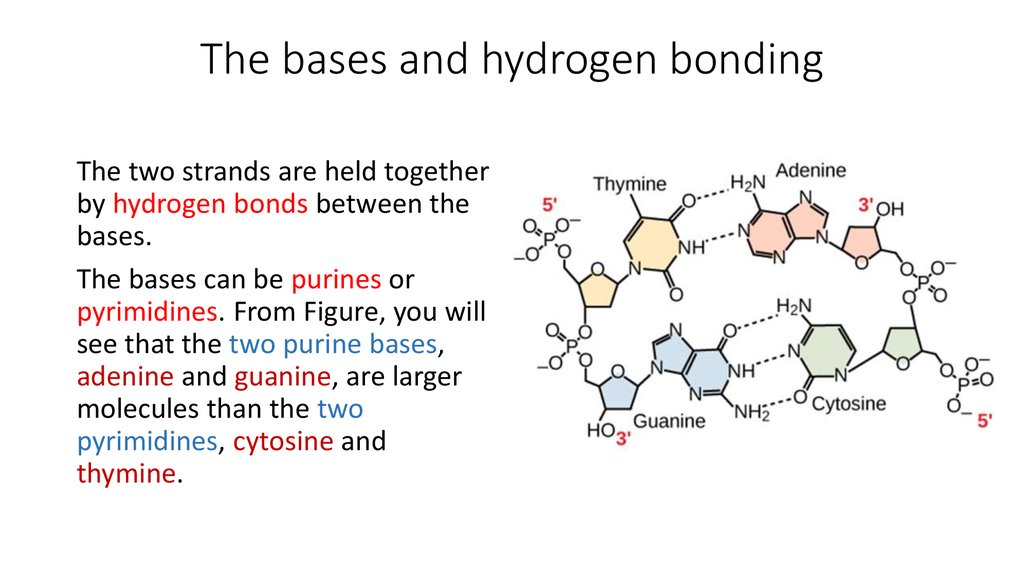
10. The bases and hydrogen bonding
The two strands are held togetherby hydrogen bonds between the
bases.
The bases can be purines or
pyrimidines. From Figure, you will
see that the two purine bases,
adenine and guanine, are larger
molecules than the two
pyrimidines, cytosine and
thymine.
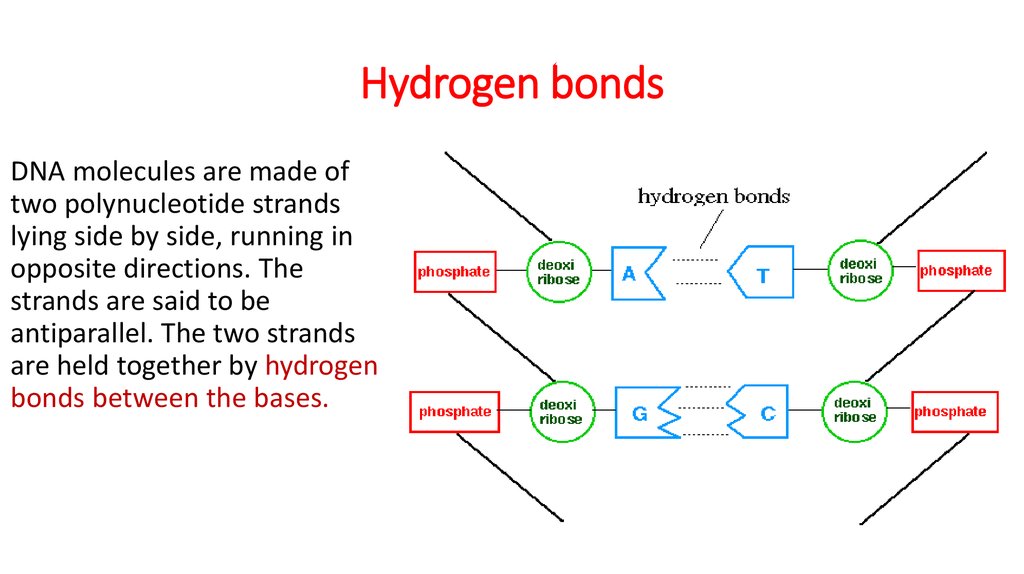
11. Hydrogen bonds
DNA molecules are made oftwo polynucleotide strands
lying side by side, running in
opposite directions. The
strands are said to be
antiparallel. The two strands
are held together by hydrogen
bonds between the bases.
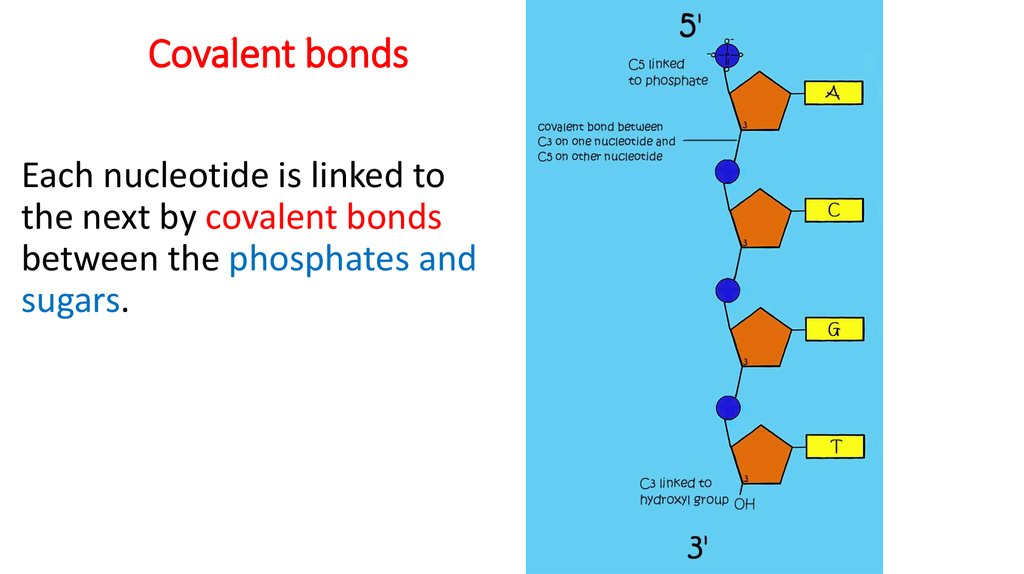
12. Covalent bonds
Each nucleotide is linked tothe next by covalent bonds
between the phosphates and
sugars.
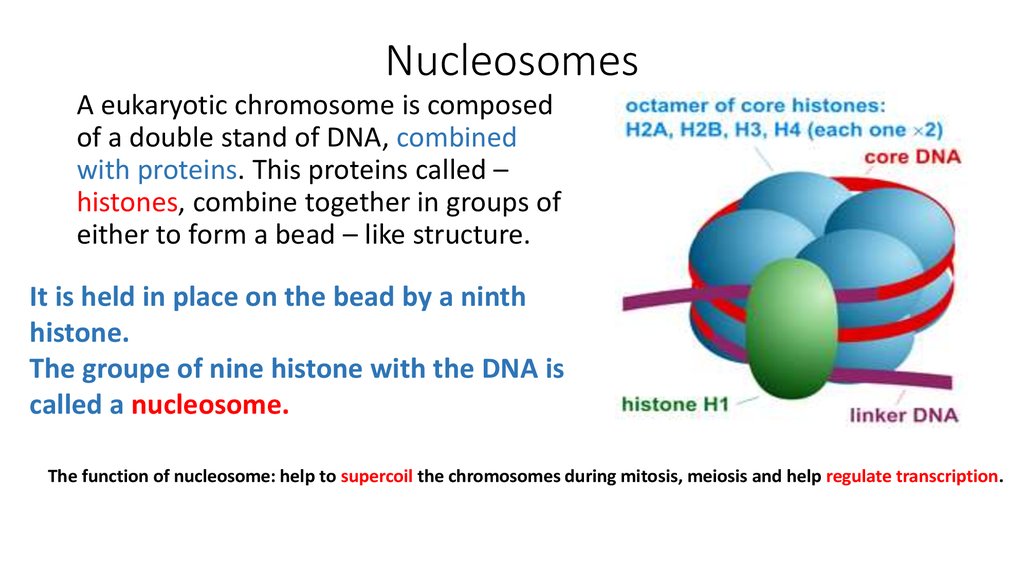
13. Nucleosomes
A eukaryotic chromosome is composedof a double stand of DNA, combined
with proteins. This proteins called –
histones, combine together in groups of
either to form a bead – like structure.
It is held in place on the bead by a ninth
histone.
The groupe of nine histone with the DNA is
called a nucleosome.
The function of nucleosome: help to supercoil the chromosomes during mitosis, meiosis and help regulate transcription.
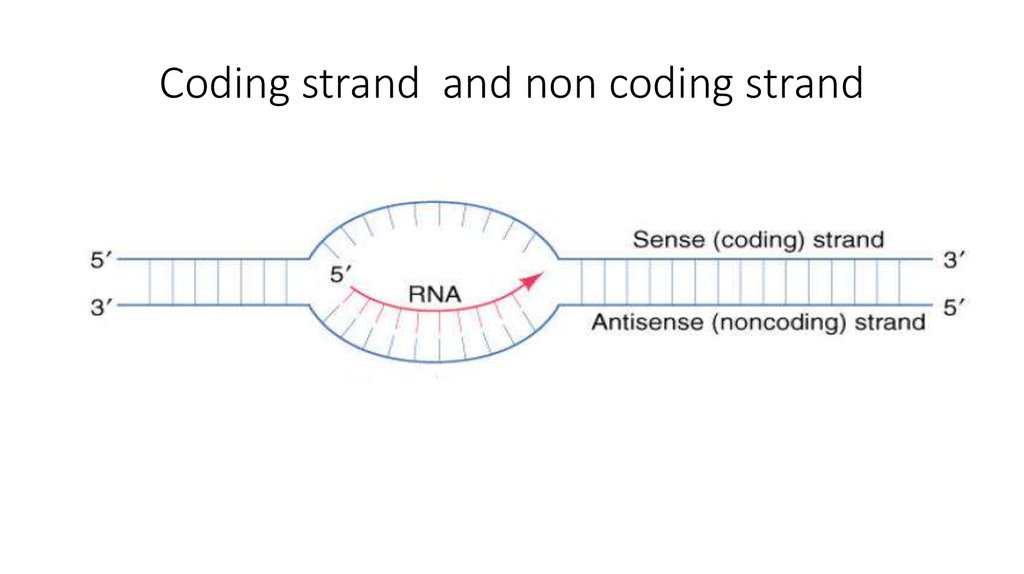
14. Coding strand and non coding strand
15.
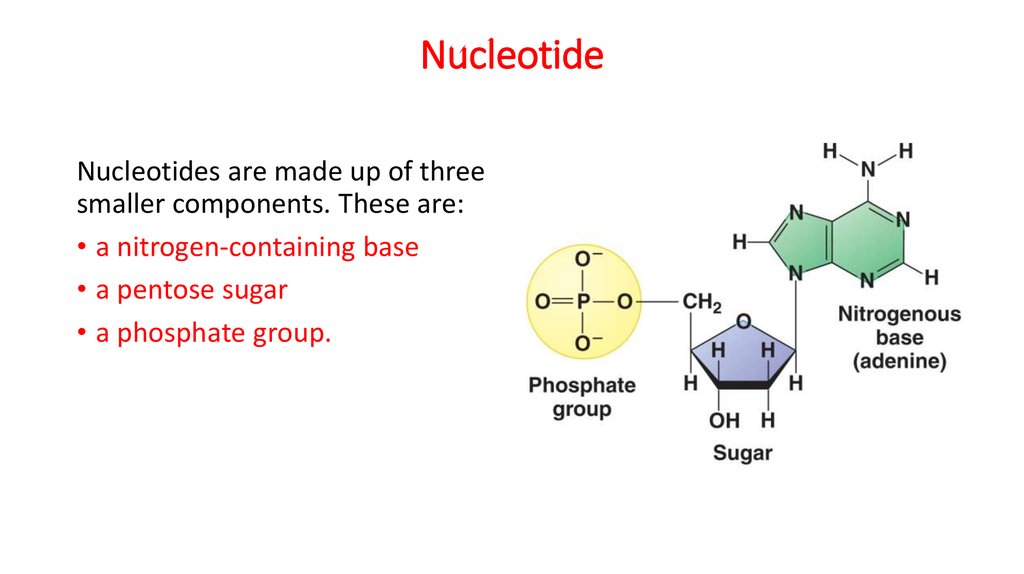
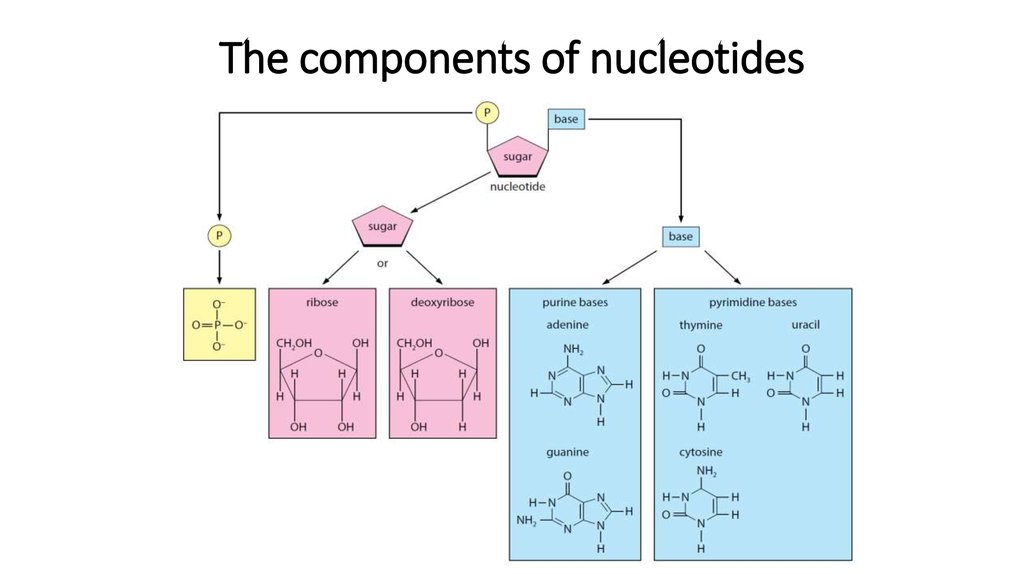
16. Nucleotide
Nucleotides are made up of threesmaller components. These are:
• a nitrogen-containing base
• a pentose sugar
• a phosphate group.
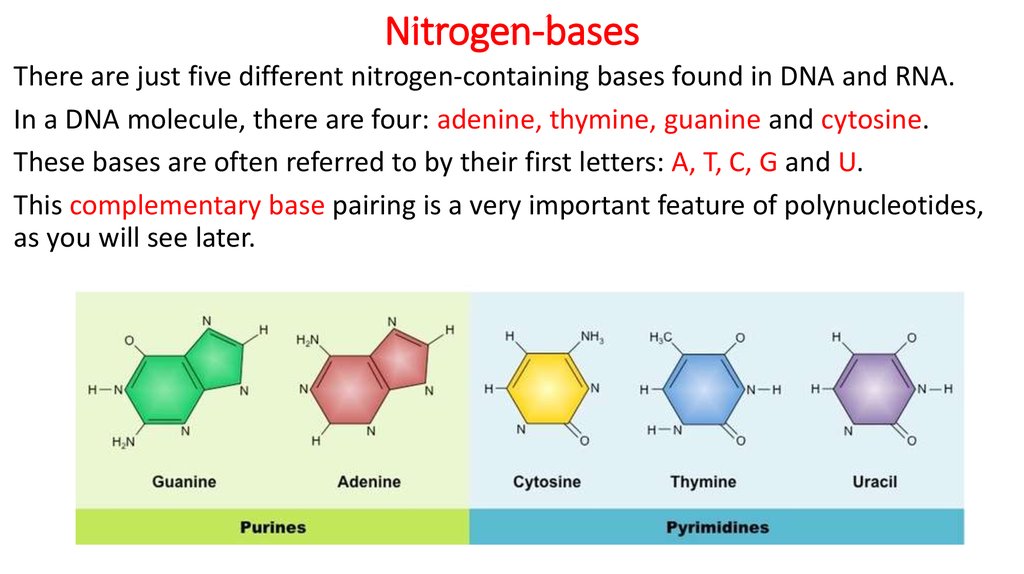
17. Nitrogen-bases
There are just five different nitrogen-containing bases found in DNA and RNA.In a DNA molecule, there are four: adenine, thymine, guanine and cytosine.
These bases are often referred to by their first letters: A, T, C, G and U.
This complementary base pairing is a very important feature of polynucleotides,
as you will see later.
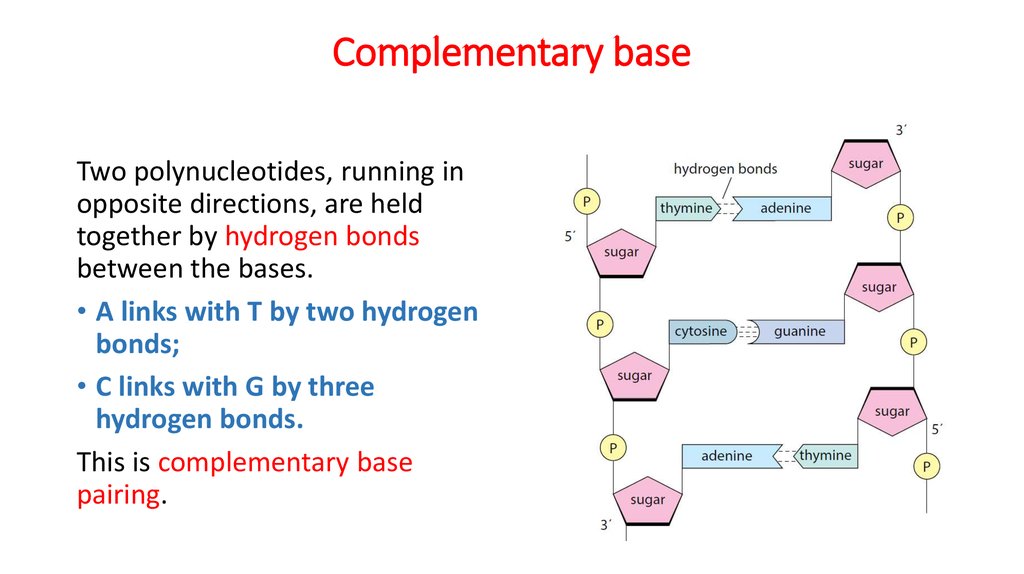
18. Complementary base
Two polynucleotides, running inopposite directions, are held
together by hydrogen bonds
between the bases.
• A links with T by two hydrogen
bonds;
• C links with G by three
hydrogen bonds.
This is complementary base
pairing.
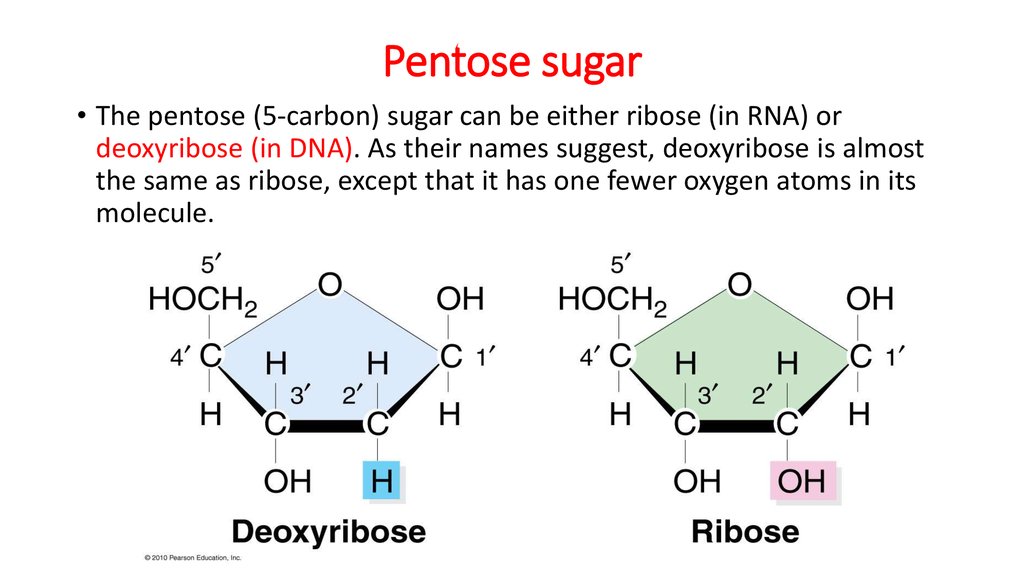
19. Pentose sugar
• The pentose (5-carbon) sugar can be either ribose (in RNA) ordeoxyribose (in DNA). As their names suggest, deoxyribose is almost
the same as ribose, except that it has one fewer oxygen atoms in its
molecule.
20.
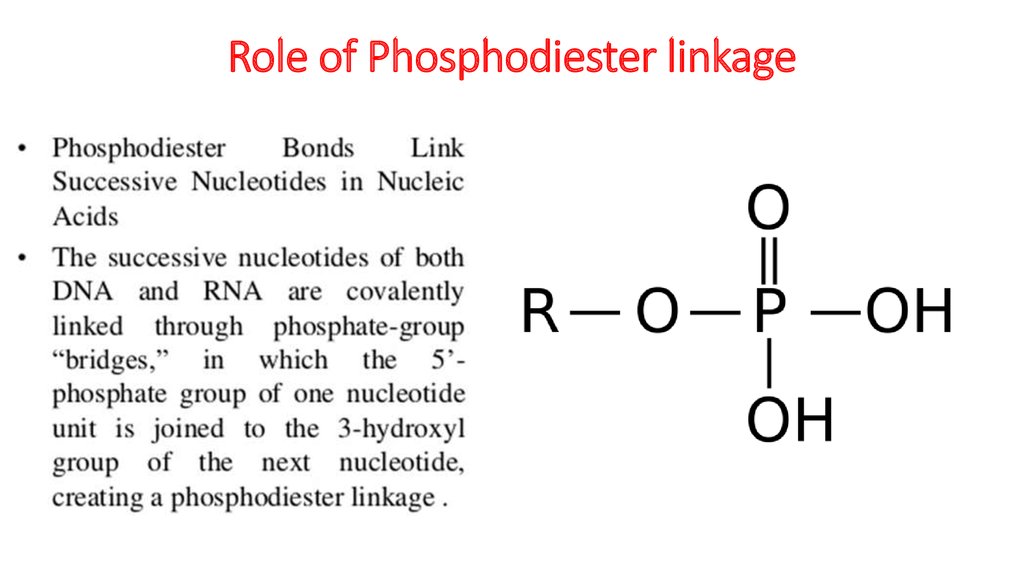
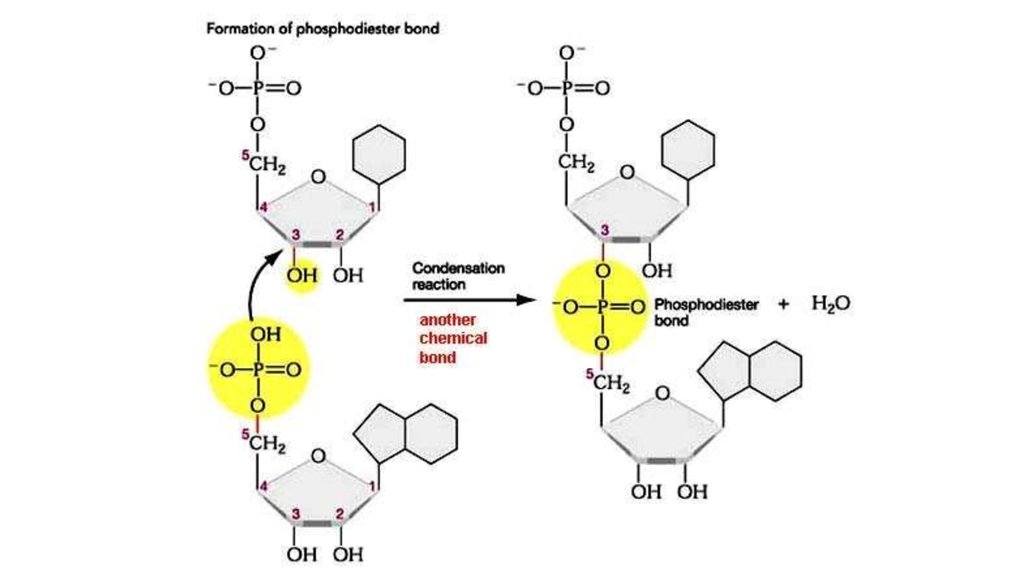
21. Role of Phosphodiester linkage
22.
23. The components of nucleotides
24.
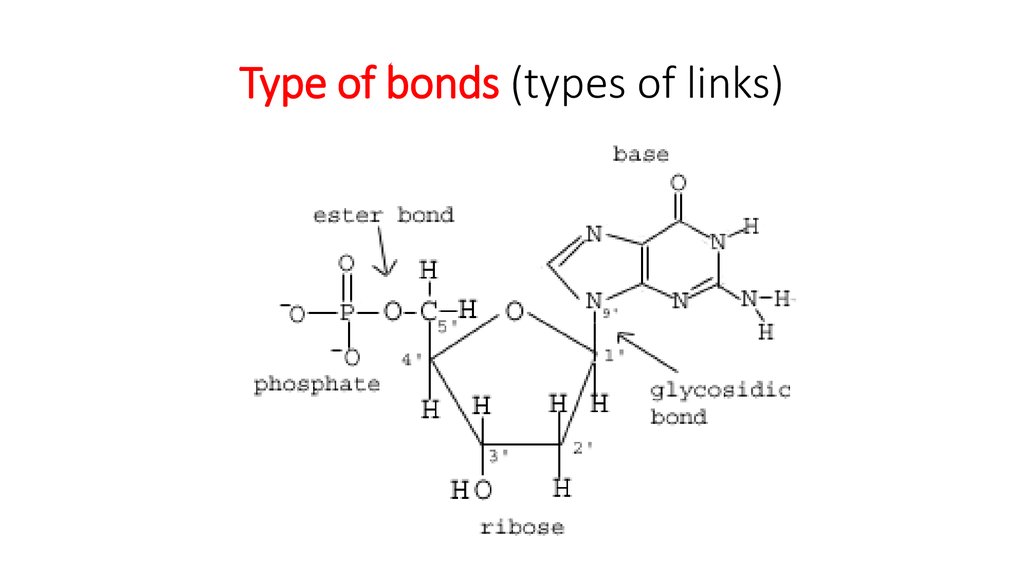
25. Type of bonds (types of links)
26.
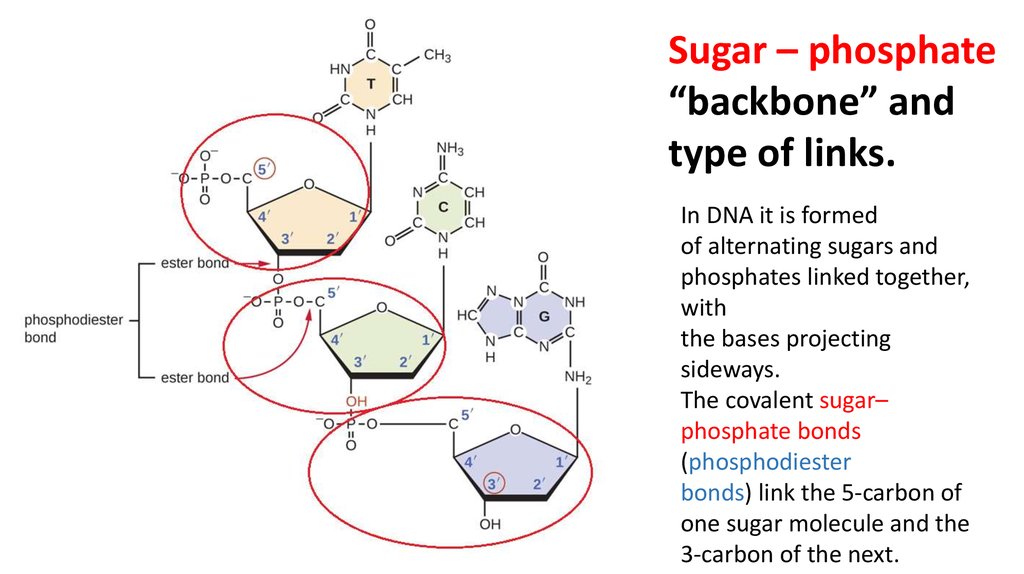
Sugar – phosphate“backbone” and
type of links.
In DNA it is formed
of alternating sugars and
phosphates linked together,
with
the bases projecting
sideways.
The covalent sugar–
phosphate bonds
(phosphodiester
bonds) link the 5-carbon of
one sugar molecule and the
3-carbon of the next.
27.
28.
29. Home task
• Read the text about the structure of DNA.• Pages: 111 – 112 “Nucleotides” and 113 “Polynucleotides”.








 biology
biology