Similar presentations:
Mmunophysiology of liver. Immunophysiology of liver functions of liver and immune system
1. Immunophysiology of liver
IMMUNOPHYSIOLOGY OF LIVERFunctions of liver and
immune system
2.
3.
4.
5.
6.
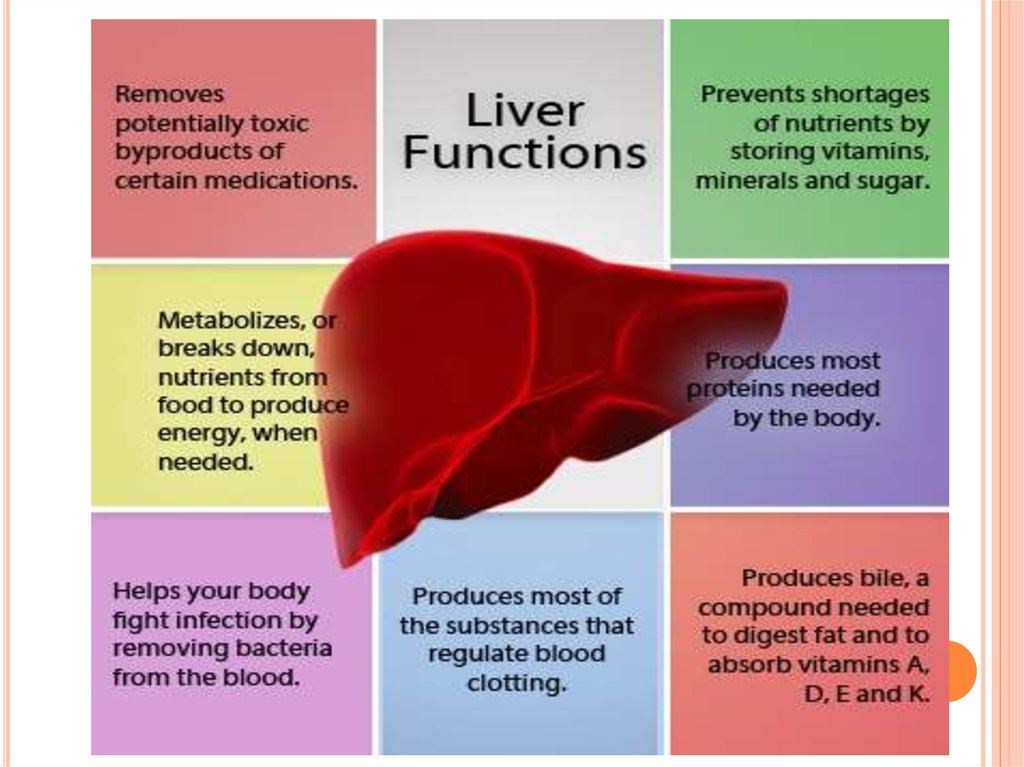
Immunological and metabolic roles of hepatocytes. Hepatocytes perform anumber of important immunological roles, in addition to their essential
metabolic roles. These include: the production of plasma proteins such as
clotting factors, complement and antimicrobial proteins; the production of
acute phase proteins upon local or systemic infection; and antigen
presentation to T cells within the liver.
7.
8.
9.
Liver and immune system can regulate metabolic and immune functions by commonregulatory molecular pathways and pathogen-sensing systems. Among these, lipidrelated pathways and TLR4–NF-κβ pathway play a major role; both are activated by
metabolic, nutritional, and immunological stimuli, and can influence and regulate
energy balance in response to changes in nutritional environment and inflammatory
status.
Many observations point out a fine balance between immune and metabolic systems,
identifying a main role for liver. The dysfunction between these two systems is unsafe
and triggers the development of several pathologies. Overnutrition and obesity impair
metabolic homeostasis, cause stress, and arise inflammatory process, contributing to
the development of the obesity-related inflammatory diseases. Conversely
undernutrition and malnutrition suppress immune system and increase susceptibility
to infections.
The condition sine qua non of NAFLD onset is macrovesicular steatosis or fatty liver,
characterized by cellular accumulation of fat, mainly in the form of triglycerides, and
sustained and amplified by the inflammatory process.
10.
11.
Most cytokine research on obesity-relateddiseases has centered on IL-6, which was among
the first cytokine to be implicated as a predictor or
pathogenetic marker of Insulin Resistance (IR)
and cardiovascular disease. This cytokine plays a
key role in the onset of hepatic IR, which was
found reduced in obese mice on high fat diet
treated with anti-IL-6 antibodies.
A definite answer to the role of IL-6 in IR will be
only possible when more clinical data will be
available on the use of IL-6-neutralizing antibody
in diabetic and/or IR patients. To date only two
small clinical trials, however, suggest a beneficial
effect.
12.
13.
14.
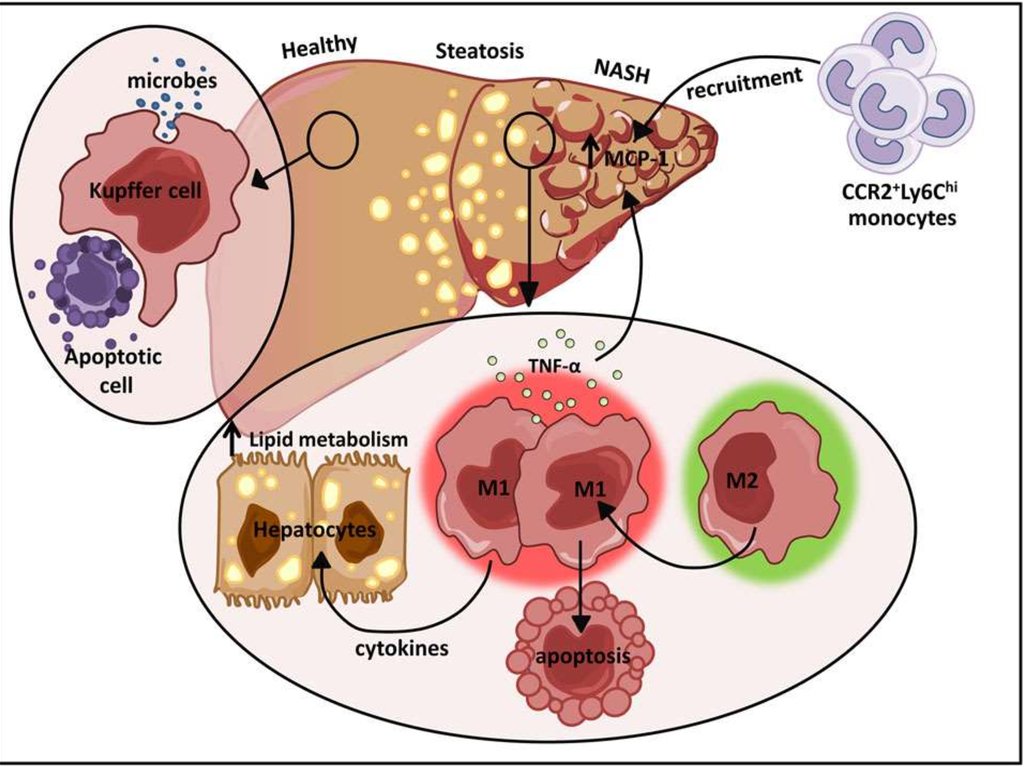
Figure. Hypothesis explaining the progression of NAFLD/NASH.Overnutrition or inactivity leads to adipocyte hypertrophy and
dysfunction, which are linked to chronic inflammation and insulin
resistance through the recruitment and activation of immune cells such
as macrophages and T-cells.
Excess fat intake and obesity lead to hyperglycemia, hyperlipidemia,
and the oversecretion of adipocytokines and the chemokines tumor
necrosis factor (TNF)-a, interleukin (IL)-1, and monocyte
chemoattractant protein (MCP)-1/C-C chemokine ligand 2 (CCL2).
These factors further contribute to the development of systemic insulin
resistance and hepatic steatosis. The latter causes hepatic
inflammation and induces NASH and even cirrhosis. Hepatic
inflammation involves the recruitment of macrophages/Kupffer cells
and an M1-dominant phenotypic shift in macrophages in the liver,
activating hepatic stellate cells and finally leading to liver fibrosis.
15.
16.
In addition to the perisinusoidal stellate cells, the KC constitute a cellular component of the hepaticsinusoids, being anchored to the luminal site of the endothelium and, thus, exposed to the
bloodstream. In contrast to stellate cells, which are distributed almost homogeneously throughout the
different zones of the liver lobule, the majority of KC is found in periportal regions where they are
larger and have greater phagocytic activity than those located in the perilobular region. This results in
a zonal distribution with a specific kinetics of phagocytosis. KC are attached to the endothelium by
cytoplasmic processes, which sometimes also anchor across the lumen to the opposite sinusoidal wall.
By their large bodies protruding into the sinusoidal lumen, KC represent a flow hindrance and are
considered as contractile cells contributing to blood flow regulation through sinusoids.
В дополнение к перисинусоидальным звездчатым клеткам Купферовские клетки (КК) представляют
собой клеточный компонент печеночных синусоид, прикрепленных к просветному участку эндотелия
и, таким образом, они подвергаются воздействию кровотока. В отличие от звездчатых клеток (stellate
cells), которые распределены почти гомогенно по разным зонам долей печени, большинство КК
обнаружено в перипортальных областях, где их больше и они имеют большую фагоцитарную
активность, чем те, которые расположены в перилобулярной области . Это приводит к зональному
распределению со специфической кинетикой фагоцитоза. КК прикреплены к эндотелию, иногда
также прикрепляются через просвет к противоположной синусоидальной стенке. По своим крупным
телам, выступающим в синусоидальный просвет, KC представляет собой препятствие потоку и
рассматривается как сократительные клетки, способствующие регуляции кровотока через
синусоиды
17.
18.
19.
Vasoactive agents, produced by KC (Kupffer cells)Agents
Function
1. Thromboxane A2
Vasoconstriction, platelet activation and
aggregation, leukocyte adhesion,
2. Nitric oxide
Vasodilation
3. Endothelin-1
Vasoconstriction Vasodilation
4. Carbon monoxide
Vasodilation
20.
21.
Cytokine signaling during liverregeneration. TNFa action on Kupffer
cells through the TNF receptor 1
(TNFR1) results in activation of NFkB and production and release of IL6. Both of these cytokines can act on
hepatocytes. Induction of NF- kB by
TNF-a in these cells leads to the
transcription of genes involved in
both cell growth and survival. IL-6
acts through the IL-6R/gp130
complex
to
stimulate
several
signaling pathways. MAPK activity
leads cell growth, while STAT3
additionally induces anti-apoptotic
gene expression. IL-6 simultaneously
activates PI3K/Akt to further promote
hepatoprotection.















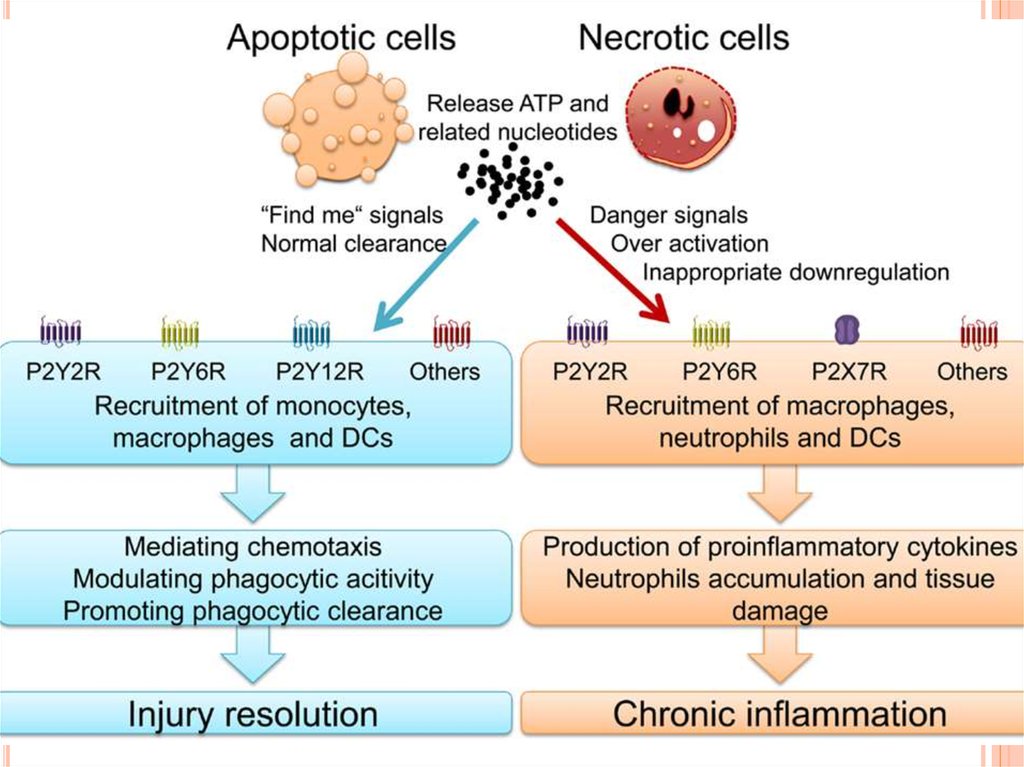

 medicine
medicine








