Similar presentations:
Pathological anatomy of damage - pathology of the
1. Pathological anatomy of damage - pathology of the cell (nucleus and cytoplasm).
Abuov Alau 267Pathological anatomy
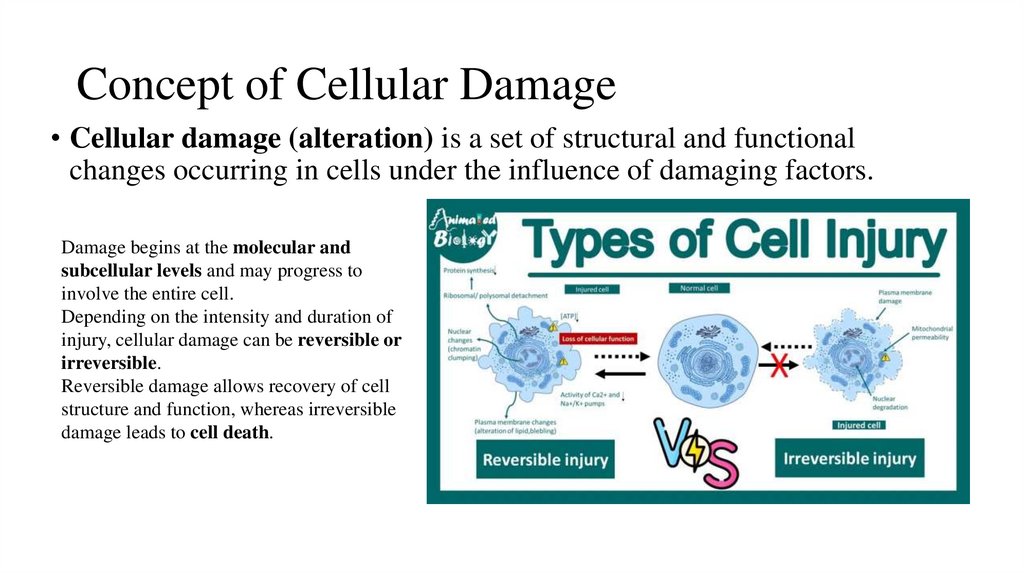
2. Concept of Cellular Damage
• Cellular damage (alteration) is a set of structural and functionalchanges occurring in cells under the influence of damaging factors.
Damage begins at the molecular and
subcellular levels and may progress to
involve the entire cell.
Depending on the intensity and duration of
injury, cellular damage can be reversible or
irreversible.
Reversible damage allows recovery of cell
structure and function, whereas irreversible
damage leads to cell death.
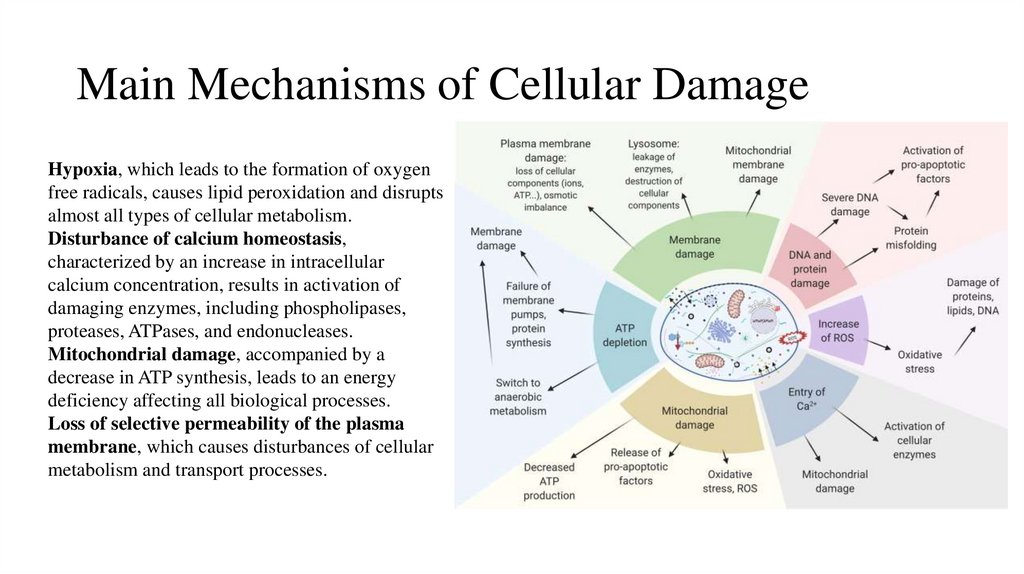
3. Main Mechanisms of Cellular Damage
Hypoxia, which leads to the formation of oxygenfree radicals, causes lipid peroxidation and disrupts
almost all types of cellular metabolism.
Disturbance of calcium homeostasis,
characterized by an increase in intracellular
calcium concentration, results in activation of
damaging enzymes, including phospholipases,
proteases, ATPases, and endonucleases.
Mitochondrial damage, accompanied by a
decrease in ATP synthesis, leads to an energy
deficiency affecting all biological processes.
Loss of selective permeability of the plasma
membrane, which causes disturbances of cellular
metabolism and transport processes.
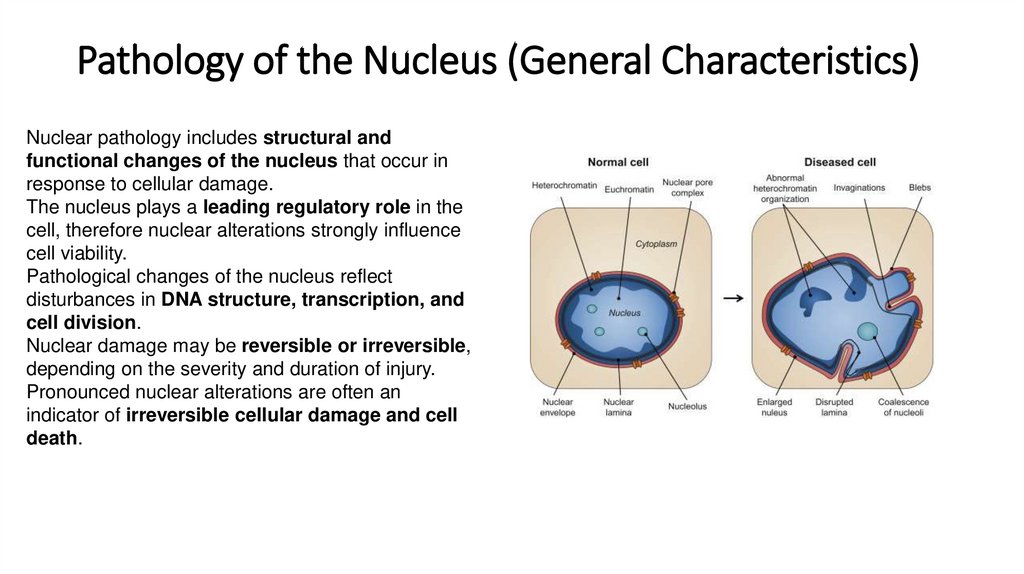
4. Pathology of the Nucleus (General Characteristics)
Nuclear pathology includes structural andfunctional changes of the nucleus that occur in
response to cellular damage.
The nucleus plays a leading regulatory role in the
cell, therefore nuclear alterations strongly influence
cell viability.
Pathological changes of the nucleus reflect
disturbances in DNA structure, transcription, and
cell division.
Nuclear damage may be reversible or irreversible,
depending on the severity and duration of injury.
Pronounced nuclear alterations are often an
indicator of irreversible cellular damage and cell
death.
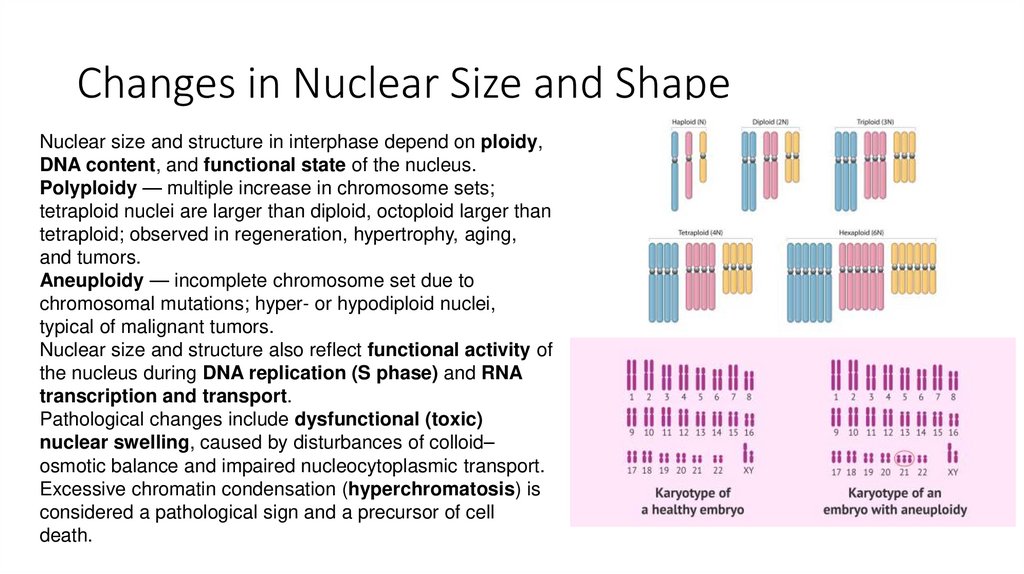
5. Changes in Nuclear Size and Shape
Nuclear size and structure in interphase depend on ploidy,DNA content, and functional state of the nucleus.
Polyploidy — multiple increase in chromosome sets;
tetraploid nuclei are larger than diploid, octoploid larger than
tetraploid; observed in regeneration, hypertrophy, aging,
and tumors.
Aneuploidy — incomplete chromosome set due to
chromosomal mutations; hyper- or hypodiploid nuclei,
typical of malignant tumors.
Nuclear size and structure also reflect functional activity of
the nucleus during DNA replication (S phase) and RNA
transcription and transport.
Pathological changes include dysfunctional (toxic)
nuclear swelling, caused by disturbances of colloid–
osmotic balance and impaired nucleocytoplasmic transport.
Excessive chromatin condensation (hyperchromatosis) is
considered a pathological sign and a precursor of cell
death.
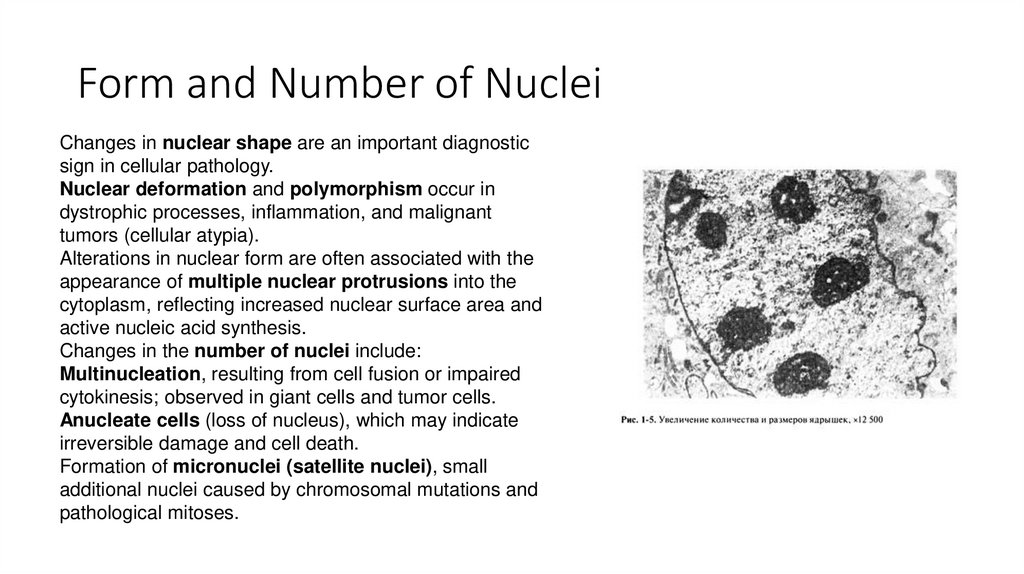
6. Form and Number of Nuclei
Changes in nuclear shape are an important diagnosticsign in cellular pathology.
Nuclear deformation and polymorphism occur in
dystrophic processes, inflammation, and malignant
tumors (cellular atypia).
Alterations in nuclear form are often associated with the
appearance of multiple nuclear protrusions into the
cytoplasm, reflecting increased nuclear surface area and
active nucleic acid synthesis.
Changes in the number of nuclei include:
Multinucleation, resulting from cell fusion or impaired
cytokinesis; observed in giant cells and tumor cells.
Anucleate cells (loss of nucleus), which may indicate
irreversible damage and cell death.
Formation of micronuclei (satellite nuclei), small
additional nuclei caused by chromosomal mutations and
pathological mitoses.
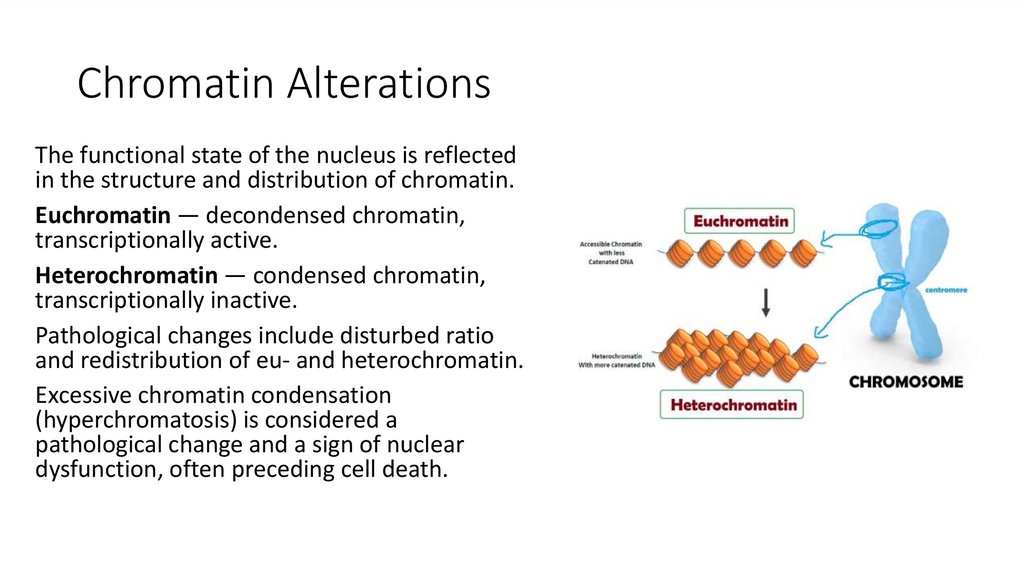
7. Chromatin Alterations
The functional state of the nucleus is reflectedin the structure and distribution of chromatin.
Euchromatin — decondensed chromatin,
transcriptionally active.
Heterochromatin — condensed chromatin,
transcriptionally inactive.
Pathological changes include disturbed ratio
and redistribution of eu- and heterochromatin.
Excessive chromatin condensation
(hyperchromatosis) is considered a
pathological change and a sign of nuclear
dysfunction, often preceding cell death.
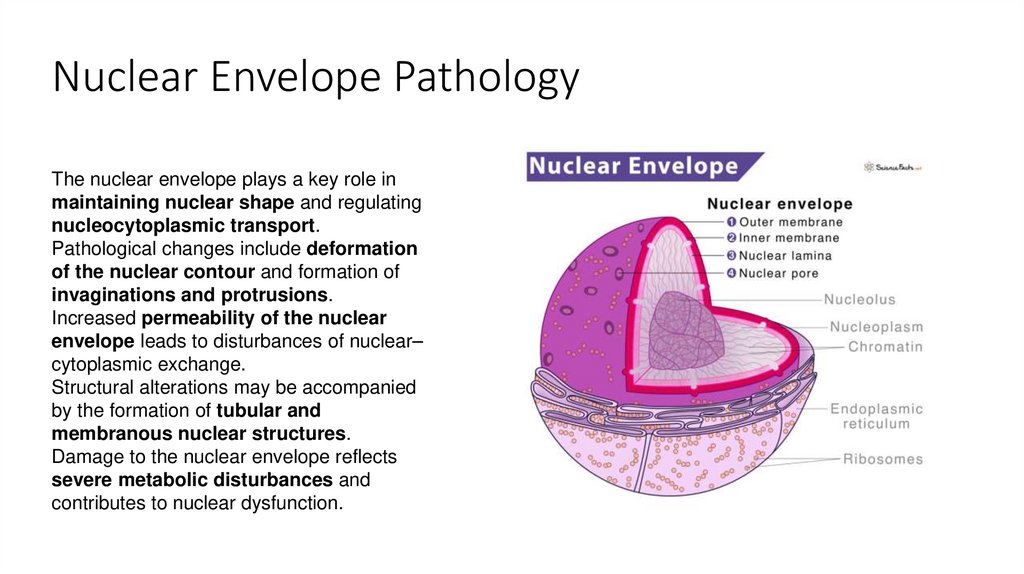
8. Nuclear Envelope Pathology
The nuclear envelope plays a key role inmaintaining nuclear shape and regulating
nucleocytoplasmic transport.
Pathological changes include deformation
of the nuclear contour and formation of
invaginations and protrusions.
Increased permeability of the nuclear
envelope leads to disturbances of nuclear–
cytoplasmic exchange.
Structural alterations may be accompanied
by the formation of tubular and
membranous nuclear structures.
Damage to the nuclear envelope reflects
severe metabolic disturbances and
contributes to nuclear dysfunction.
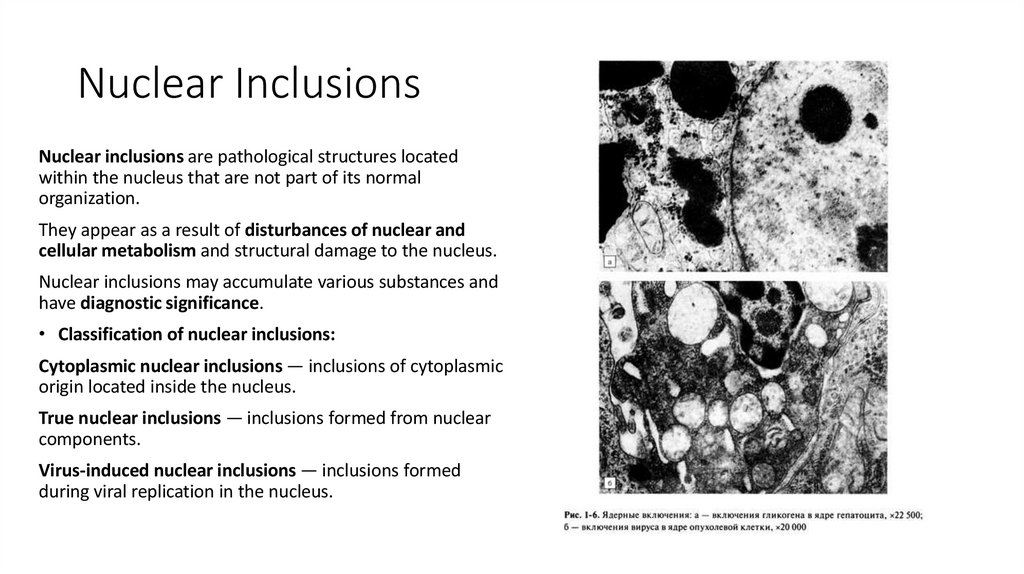
9. Nuclear Inclusions
Nuclear inclusions are pathological structures locatedwithin the nucleus that are not part of its normal
organization.
They appear as a result of disturbances of nuclear and
cellular metabolism and structural damage to the nucleus.
Nuclear inclusions may accumulate various substances and
have diagnostic significance.
• Classification of nuclear inclusions:
Cytoplasmic nuclear inclusions — inclusions of cytoplasmic
origin located inside the nucleus.
True nuclear inclusions — inclusions formed from nuclear
components.
Virus-induced nuclear inclusions — inclusions formed
during viral replication in the nucleus.
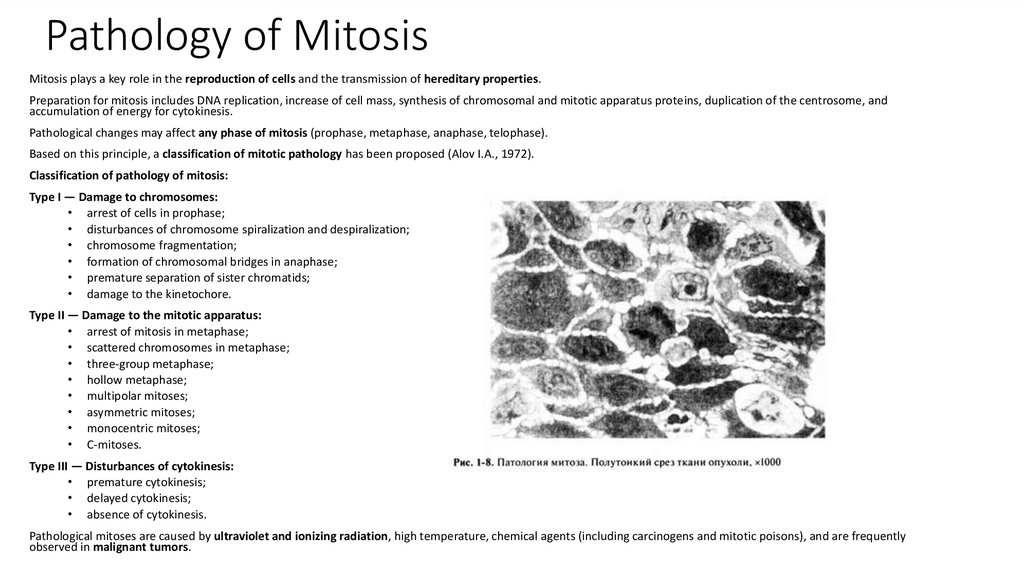
10. Pathology of Mitosis
Mitosis plays a key role in the reproduction of cells and the transmission of hereditary properties.Preparation for mitosis includes DNA replication, increase of cell mass, synthesis of chromosomal and mitotic apparatus proteins, duplication of the centrosome, and
accumulation of energy for cytokinesis.
Pathological changes may affect any phase of mitosis (prophase, metaphase, anaphase, telophase).
Based on this principle, a classification of mitotic pathology has been proposed (Alov I.A., 1972).
Classification of pathology of mitosis:
Type I — Damage to chromosomes:
• arrest of cells in prophase;
• disturbances of chromosome spiralization and despiralization;
• chromosome fragmentation;
• formation of chromosomal bridges in anaphase;
• premature separation of sister chromatids;
• damage to the kinetochore.
Type II — Damage to the mitotic apparatus:
• arrest of mitosis in metaphase;
• scattered chromosomes in metaphase;
• three-group metaphase;
• hollow metaphase;
• multipolar mitoses;
• asymmetric mitoses;
• monocentric mitoses;
• C-mitoses.
Type III — Disturbances of cytokinesis:
• premature cytokinesis;
• delayed cytokinesis;
• absence of cytokinesis.
Pathological mitoses are caused by ultraviolet and ionizing radiation, high temperature, chemical agents (including carcinogens and mitotic poisons), and are frequently
observed in malignant tumors.
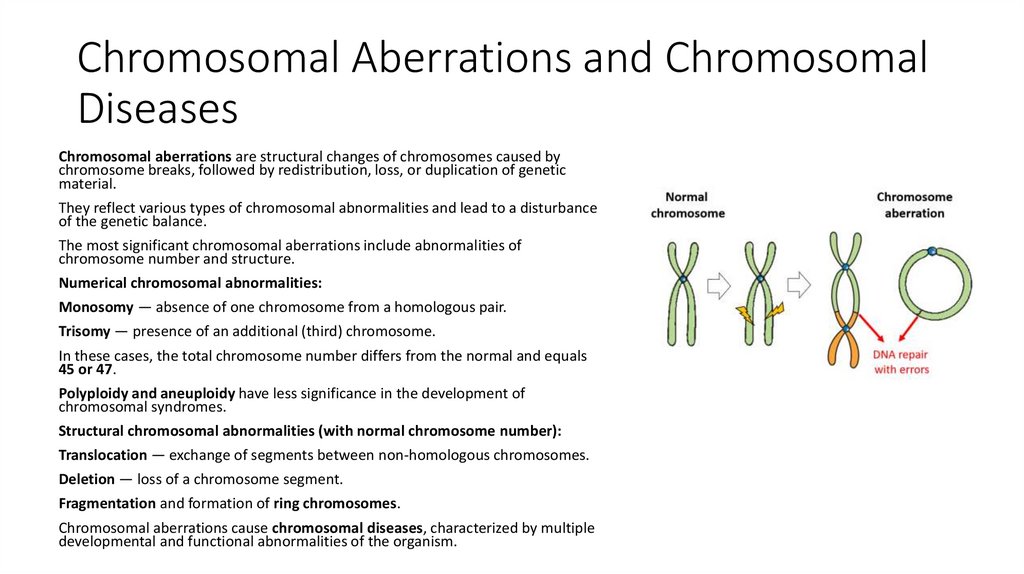
11. Chromosomal Aberrations and Chromosomal Diseases
Chromosomal aberrations are structural changes of chromosomes caused bychromosome breaks, followed by redistribution, loss, or duplication of genetic
material.
They reflect various types of chromosomal abnormalities and lead to a disturbance
of the genetic balance.
The most significant chromosomal aberrations include abnormalities of
chromosome number and structure.
Numerical chromosomal abnormalities:
Monosomy — absence of one chromosome from a homologous pair.
Trisomy — presence of an additional (third) chromosome.
In these cases, the total chromosome number differs from the normal and equals
45 or 47.
Polyploidy and aneuploidy have less significance in the development of
chromosomal syndromes.
Structural chromosomal abnormalities (with normal chromosome number):
Translocation — exchange of segments between non-homologous chromosomes.
Deletion — loss of a chromosome segment.
Fragmentation and formation of ring chromosomes.
Chromosomal aberrations cause chromosomal diseases, characterized by multiple
developmental and functional abnormalities of the organism.
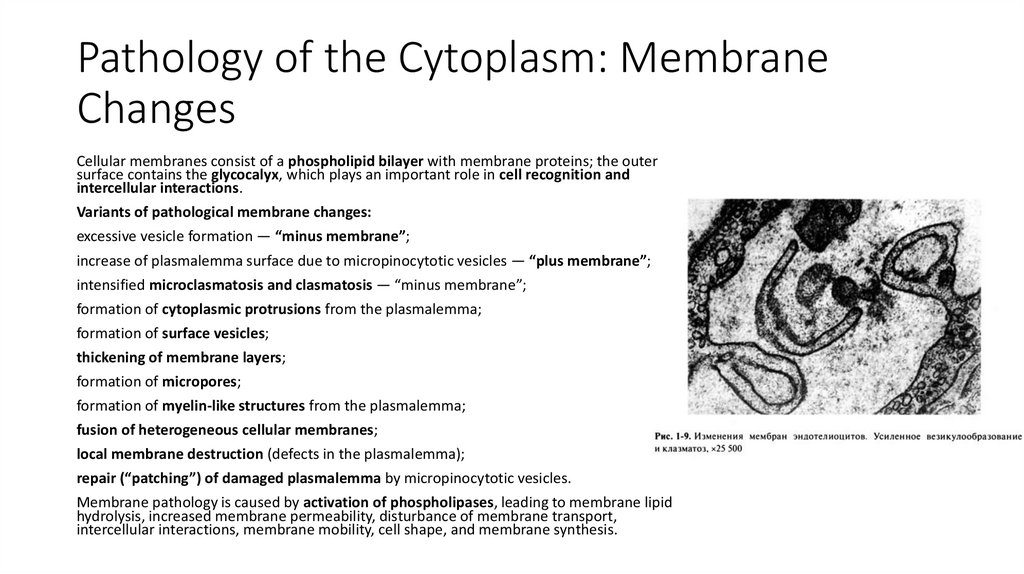
12. Pathology of the Cytoplasm: Membrane Changes
Cellular membranes consist of a phospholipid bilayer with membrane proteins; the outersurface contains the glycocalyx, which plays an important role in cell recognition and
intercellular interactions.
Variants of pathological membrane changes:
excessive vesicle formation — “minus membrane”;
increase of plasmalemma surface due to micropinocytotic vesicles — “plus membrane”;
intensified microclasmatosis and clasmatosis — “minus membrane”;
formation of cytoplasmic protrusions from the plasmalemma;
formation of surface vesicles;
thickening of membrane layers;
formation of micropores;
formation of myelin-like structures from the plasmalemma;
fusion of heterogeneous cellular membranes;
local membrane destruction (defects in the plasmalemma);
repair (“patching”) of damaged plasmalemma by micropinocytotic vesicles.
Membrane pathology is caused by activation of phospholipases, leading to membrane lipid
hydrolysis, increased membrane permeability, disturbance of membrane transport,
intercellular interactions, membrane mobility, cell shape, and membrane synthesis.
13. Disturbances of Membrane Transport
•Membrane transport ensures the movement of ions and substrates across the membrane by active (ATP-dependent) and passive(diffusion and exchange) mechanisms.
•Active transport requires ATP and mobility of membrane transport proteins and is a key function of epithelial barriers.
Disturbances of membrane transport are clearly demonstrated during ischemia, which causes primary damage to mitochondria.
During ischemia:
•oxidative phosphorylation sharply decreases;
•mitochondria swell and membrane permeability increases;
•damage later becomes total and irreversible.
•Ischemic mitochondrial injury leads to failure of the Na⁺/K⁺-ATPase, resulting in:
•accumulation of sodium in the cell;
•loss of potassium;
•displacement of calcium from mitochondria.
•Increased cytoplasmic ionized calcium and formation of calcium–calmodulin complexes cause:
•disruption of cell contacts;
•activation of phospholipases;
•cytoplasmic structural changes.
Enhanced glycolysis leads to glycogen depletion, lactate accumulation, and decrease of intracellular pH, resulting in reduced RNA
synthesis.
Irreversible ischemic damage is associated with phospholipase-mediated hydrolysis of membrane lipids and release of lysosomal
hydrolases.
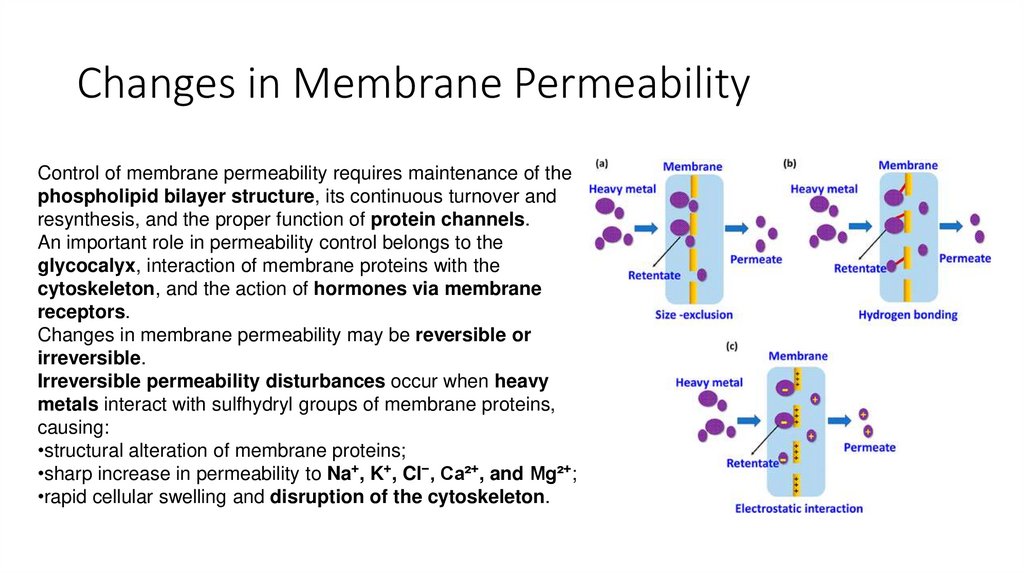
14. Changes in Membrane Permeability
Control of membrane permeability requires maintenance of thephospholipid bilayer structure, its continuous turnover and
resynthesis, and the proper function of protein channels.
An important role in permeability control belongs to the
glycocalyx, interaction of membrane proteins with the
cytoskeleton, and the action of hormones via membrane
receptors.
Changes in membrane permeability may be reversible or
irreversible.
Irreversible permeability disturbances occur when heavy
metals interact with sulfhydryl groups of membrane proteins,
causing:
•structural alteration of membrane proteins;
•sharp increase in permeability to Na⁺, K⁺, Cl⁻, Ca²⁺, and Mg²⁺;
•rapid cellular swelling and disruption of the cytoskeleton.
15. Changes in Cell Interaction and Recognition
Cell interaction and recognition of self and non-self are essential properties of cellularcooperation.
These processes are primarily determined by differences in the external surfaces of the plasma
membrane and membranes of intracellular structures.
A key role in cell recognition belongs to the membrane glycocalyx, which contains surface
antigens serving as markers of specific cell types.
Disturbances of cell interaction and recognition occur in various pathological processes, during
which surface antigens may change.
These changes concern both the type of antigen and its accessibility from the extracellular
space.
Loss of normal cell-specific antigens may be accompanied by the appearance of embryonic and
abnormal antigens (e.g., carcinoembryonic antigen).
Alterations of membrane glycolipids increase membrane susceptibility to antibody action.
Cellular communication also depends on the state of cell junctions, which may be damaged in
pathological conditions.
In tumor cells, abnormalities of cell contacts correlate with impaired intercellular connections and
the presence of abnormal cell junctions.
16. Changes in Membrane Mobility and Cell Shape
Two main types of changes related toimpaired membrane mobility are
distinguished:
• Exotropia — outward protrusion of the
membrane into the extracellular space,
forming a membrane-bound cytoplasmic
structure.
• Esotropia — inward protrusion of the
membrane into the cytoplasm, resulting in
the formation of a membrane-bound
cavity.
Changes in cell shape are caused not
only by exo- and esotropia but also by
simplification of the cell surface.
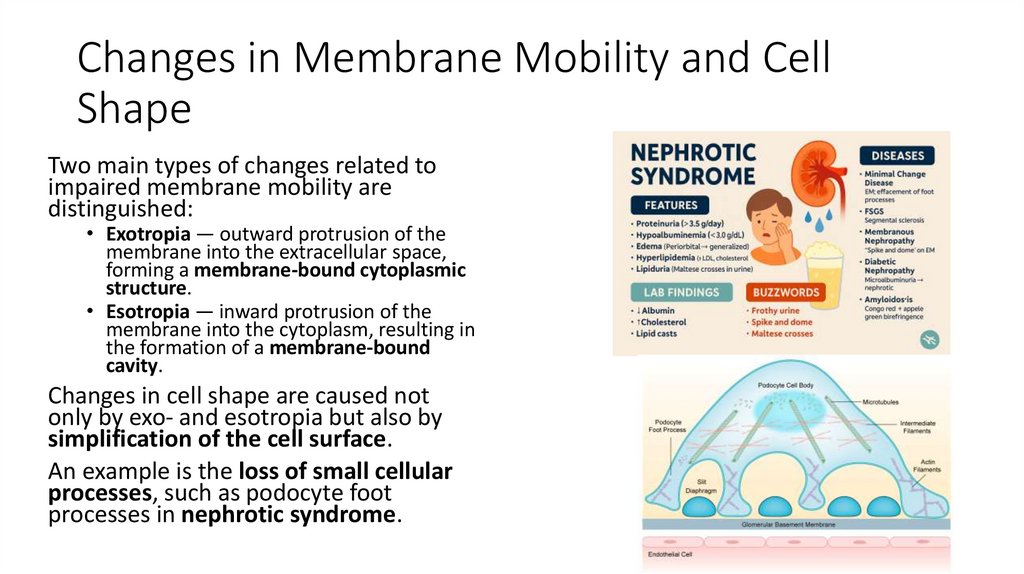
An example is the loss of small cellular
processes, such as podocyte foot
processes in nephrotic syndrome.
17. Disturbances of Membrane Synthesis and Turnover
Exposure to chemical agents may lead to either increased ordecreased membrane synthesis.
Reduced membrane synthesis is observed, for example, in enterocytes,
with decreased formation of the brush border membrane due to
inhibition of membrane enzymes.
Membrane turnover may be:
• enhanced during stimulation of autophagy;
• reduced in lysosomal storage diseases.


 biology
biology