Similar presentations:
M1_Ch1_Introduction_Day3_Recording
1. Introduction to Chemistry – Chapter 1: Chemistry Methods and Measurement
CHE-1050 – Spring 20262. 1.1 Strategies for Success in Chemistry
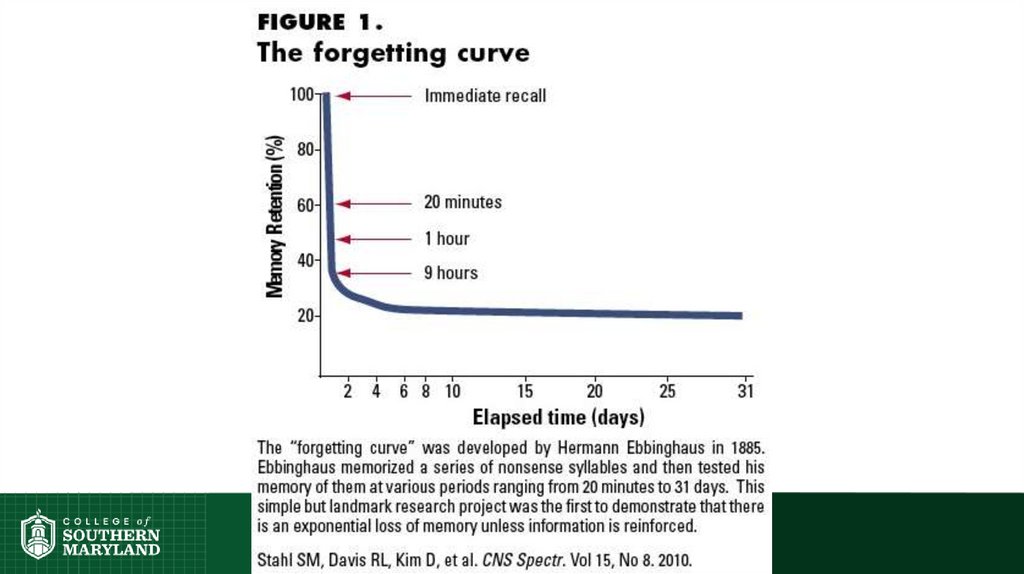
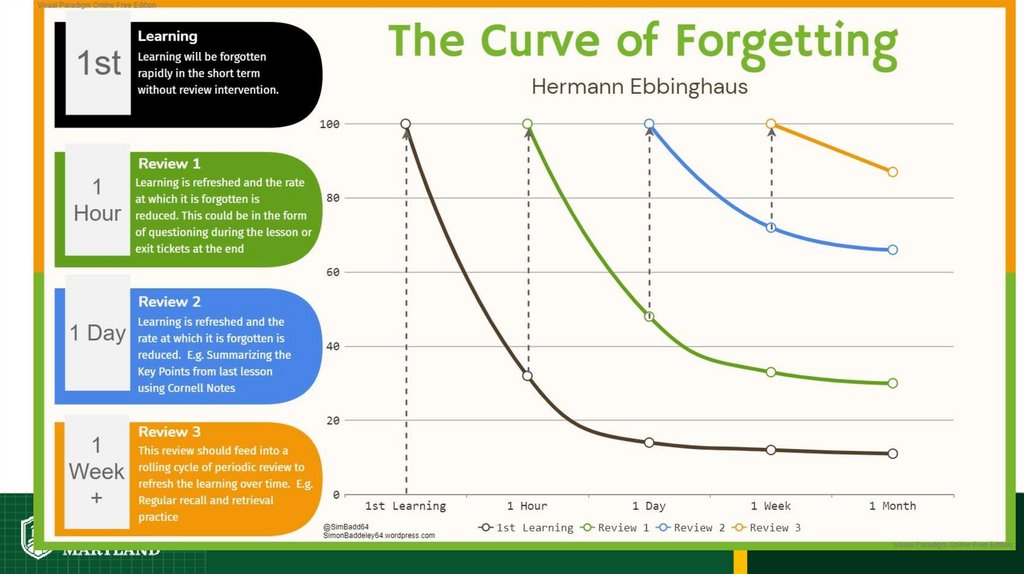
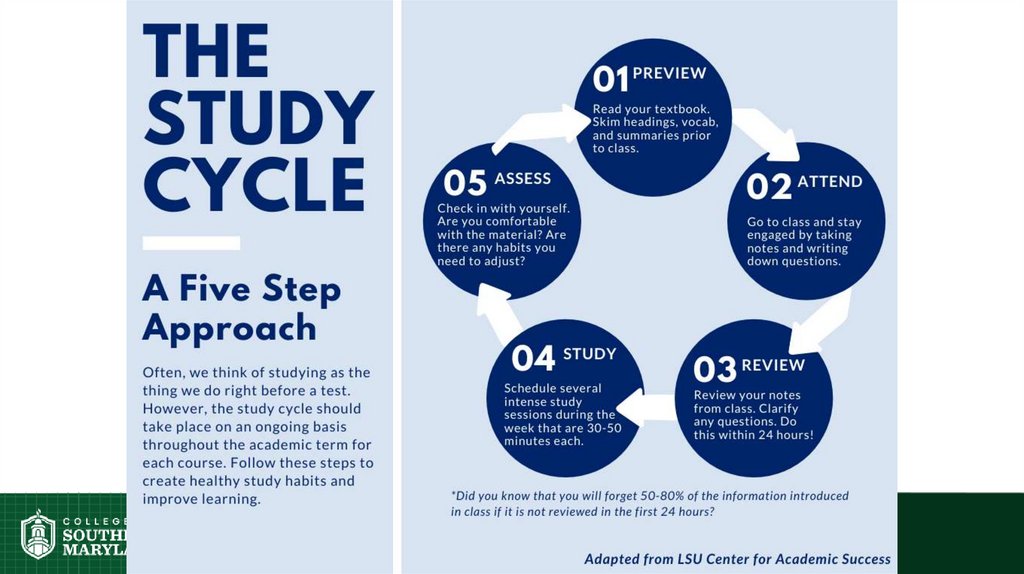
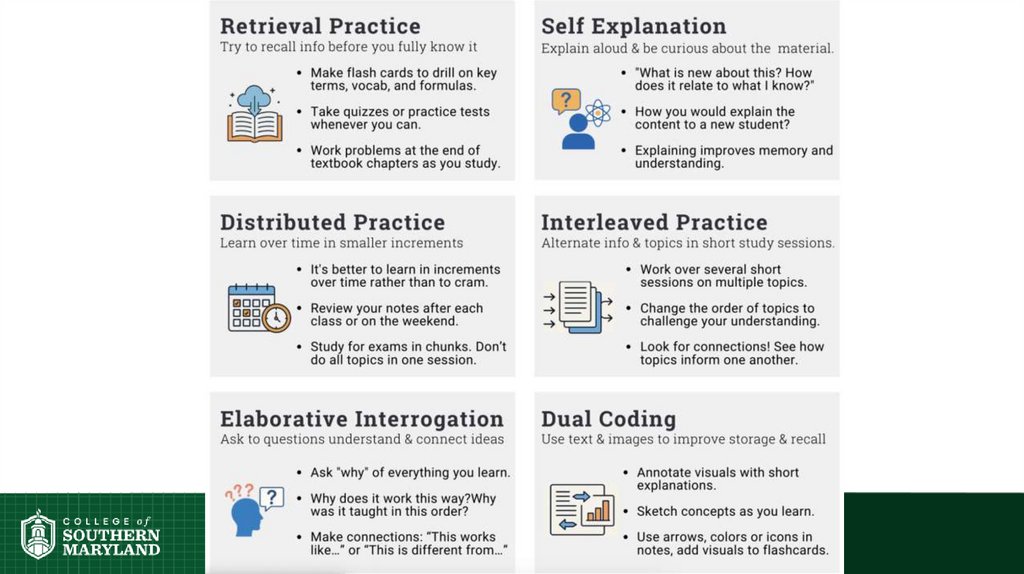
Science of Learning Chemistry:Repetition is central.
3. 1.1 Strategies for Success in Chemistry
Science of Learning Chemistry:Repetition is central.
• In physical exercise, repetition is
required to build muscle.
• In learning, repetition is required for
long-term retention of facts.
4.
5.
6.
7.
8.
9.
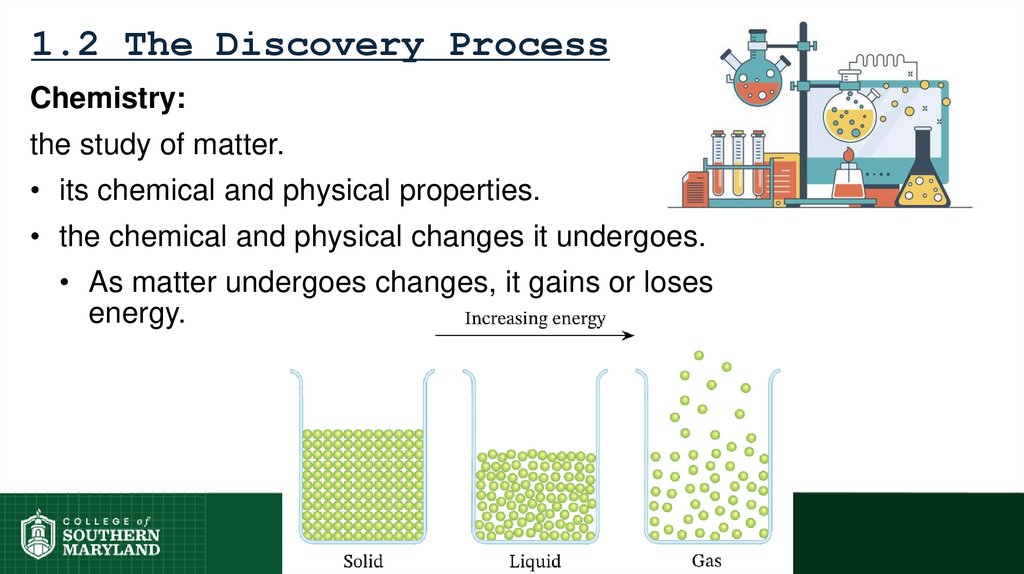
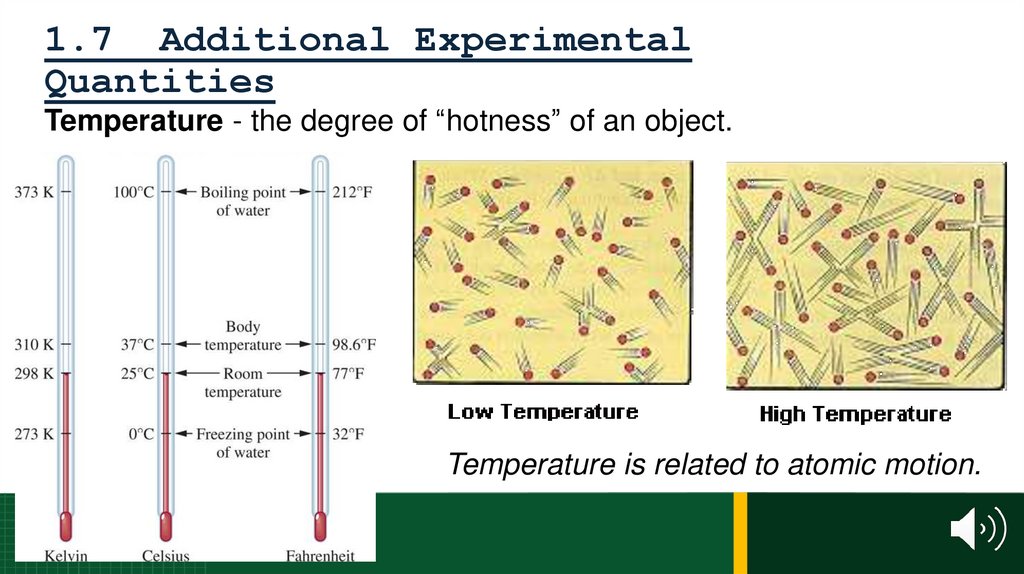
10. 1.2 The Discovery Process
Chemistry:the study of matter.
• its chemical and physical properties.
• the chemical and physical changes it undergoes.
11. 1.2 The Discovery Process
Chemistry:the study of matter.
• its chemical and physical properties.
• the chemical and physical changes it undergoes.
• As matter undergoes changes, it gains or loses
energy.
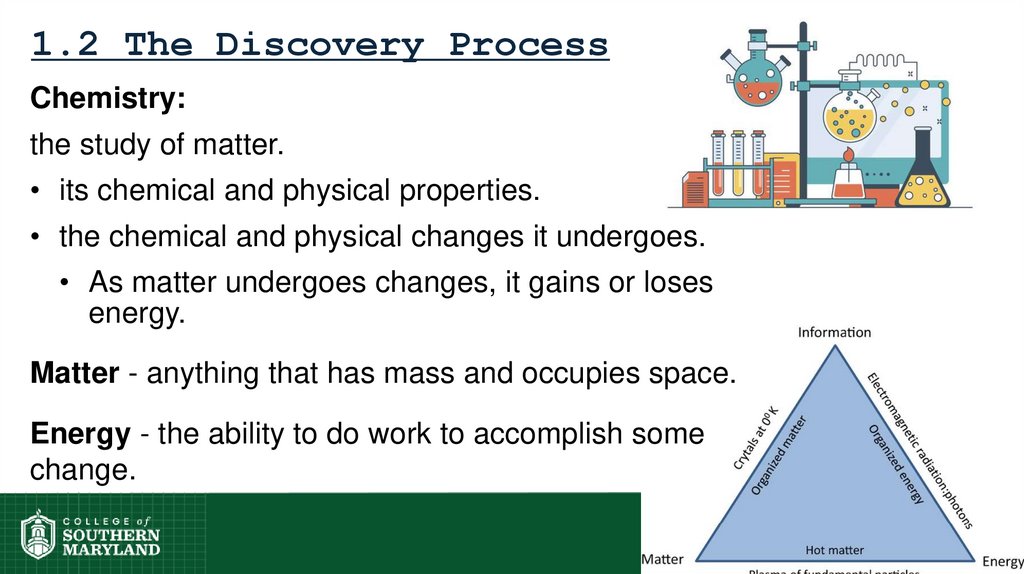
12. 1.2 The Discovery Process
Chemistry:the study of matter.
• its chemical and physical properties.
• the chemical and physical changes it undergoes.
• As matter undergoes changes, it gains or loses
energy.
Matter - anything that has mass and occupies space.
Energy - the ability to do work to accomplish some
change.
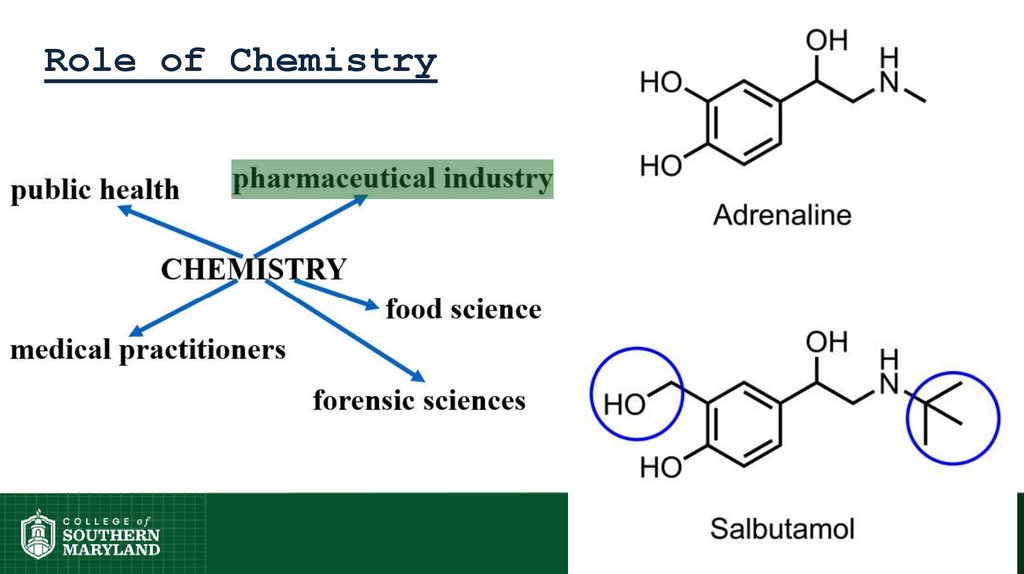
13. Role of Chemistry
14. Role of Chemistry
15. Role of Chemistry
16. Role of Chemistry
• How does the macroscaleproperties result from atomicscale properties/phenomena?
• Chemistry aspect – how do we
synthesize new materials?
17. Role of Chemistry
• How does the macroscaleproperties result from atomicscale properties/phenomena?
• Chemistry aspect – how do we
synthesize new materials?
Diamond: Hardness = 10
Resistivity ~ 1016 Ω·m
18. Role of Chemistry
• How does the macroscaleproperties result from atomicscale properties/phenomena?
• Chemistry aspect – how do we
synthesize new materials?
Diamond: Hardness = 10
Resistivity ~ 1016 Ω·m
Graphite: Hardness = 2
Resistivity ~ 10-5 Ω·m
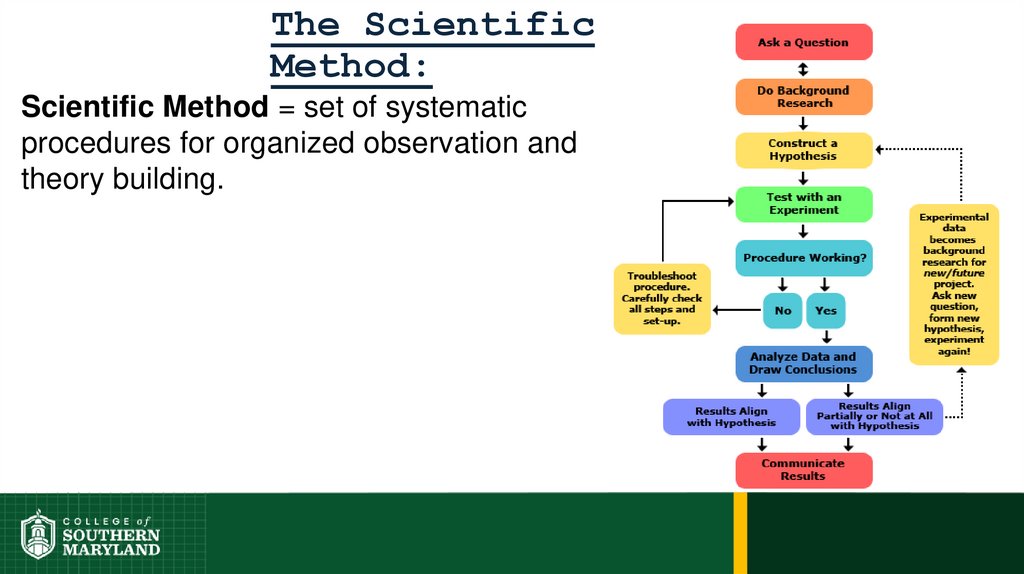
19. The Scientific Method:
Scientific Method = set of systematicprocedures for organized observation and
theory building.
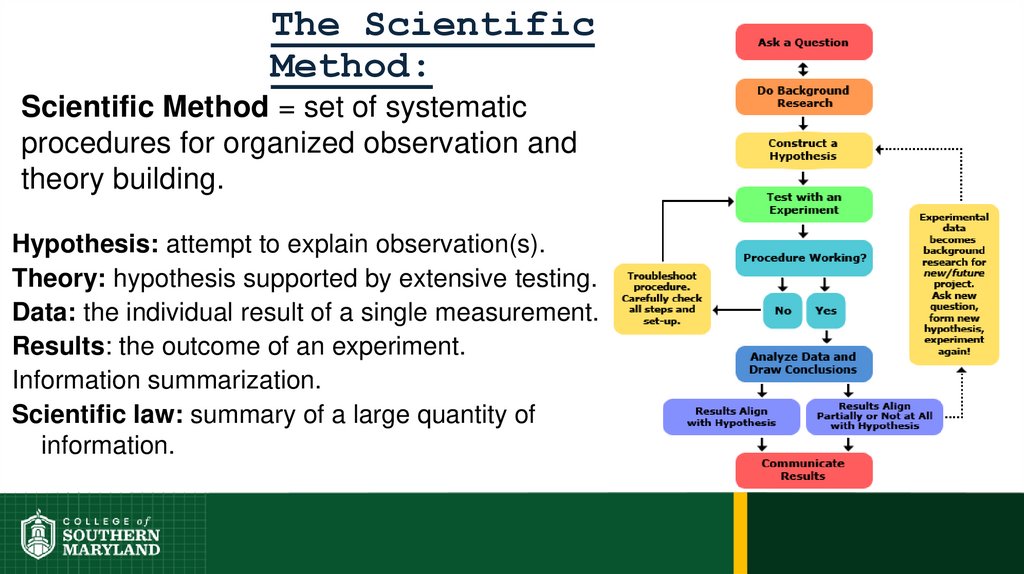
20.
The ScientificMethod:
Scientific Method = set of systematic
procedures for organized observation and
theory building.
Hypothesis: attempt to explain observation(s).
Theory: hypothesis supported by extensive testing.
Data: the individual result of a single measurement.
Results: the outcome of an experiment.
Information summarization.
Scientific law: summary of a large quantity of
information.
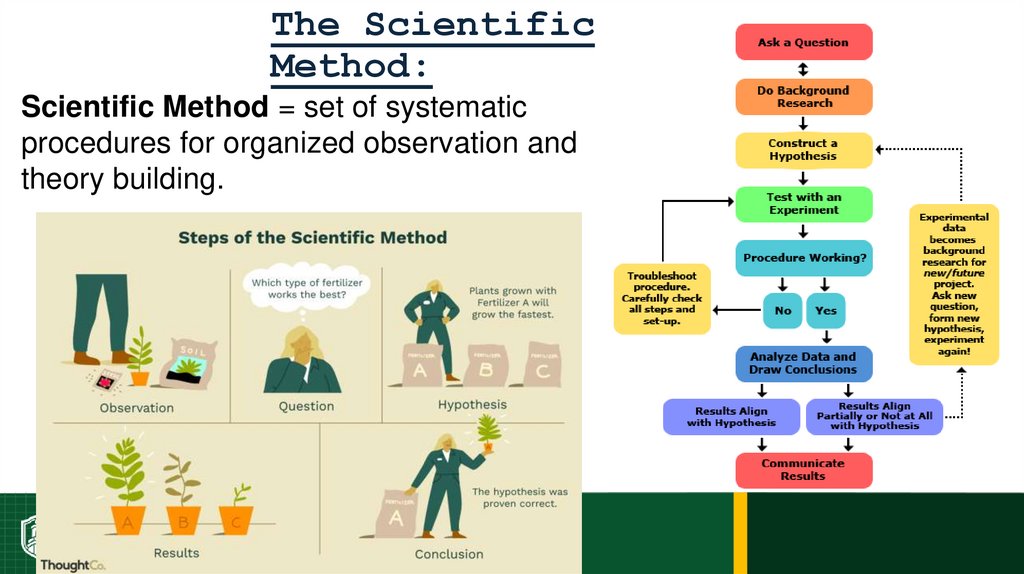
21.
The ScientificMethod:
Scientific Method = set of systematic
procedures for organized observation and
theory building.
22.
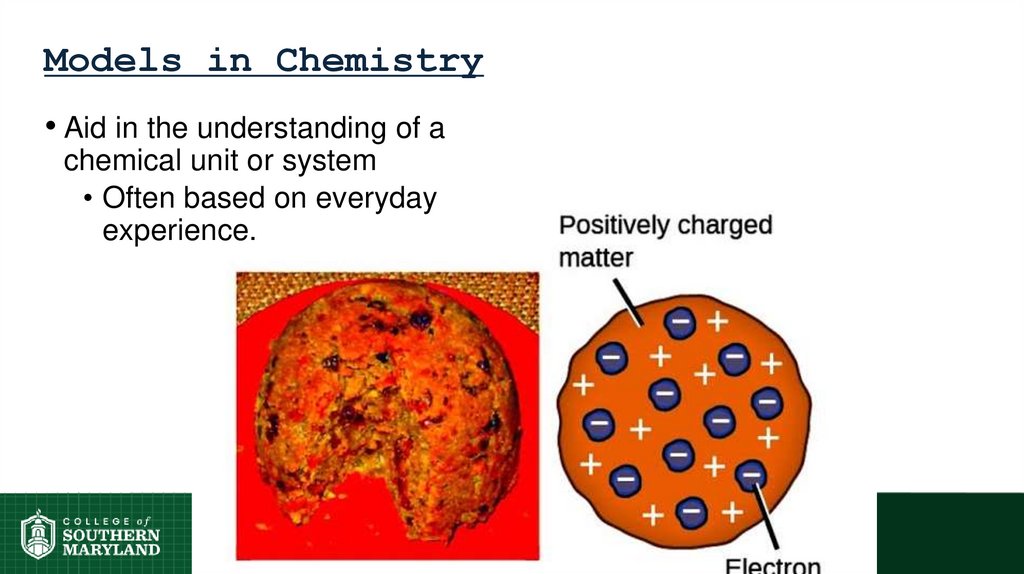
Models in Chemistry• Aid in the understanding of a
chemical unit or system
• Often based on everyday
experience.
23.
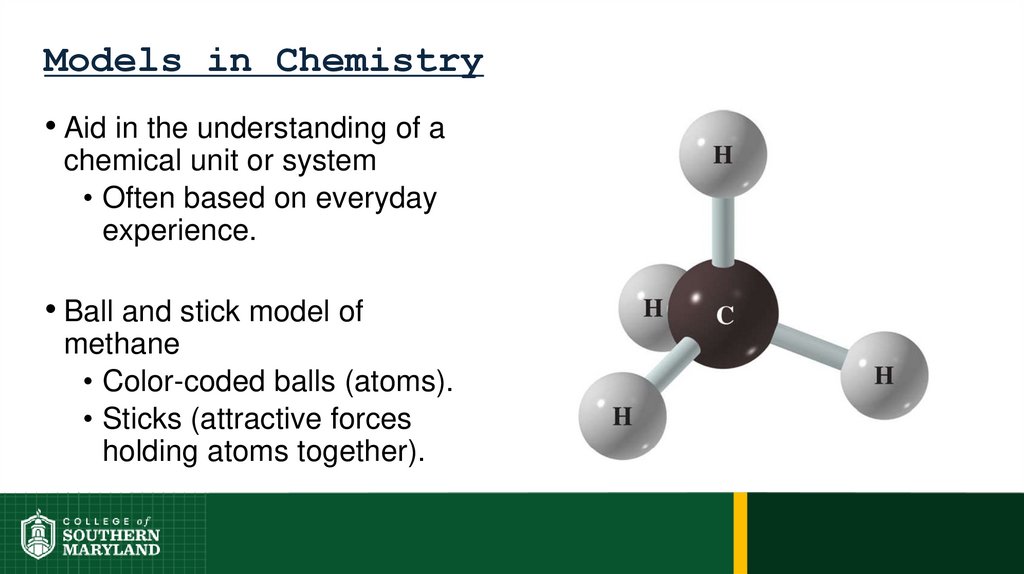
Models in Chemistry• Aid in the understanding of a
chemical unit or system
• Often based on everyday
experience.
• Ball and stick model of
methane
• Color-coded balls (atoms).
• Sticks (attractive forces
holding atoms together).
24. 1.3 The Classification of Matter
Properties - characteristics ofmatter scientists can use to
categorize different types of matter.
25. 1.3 The Classification of Matter
Properties - characteristics ofmatter scientists can use to
categorize different types of matter.
Ways to categorize matter:
1. By State
2. By Composition
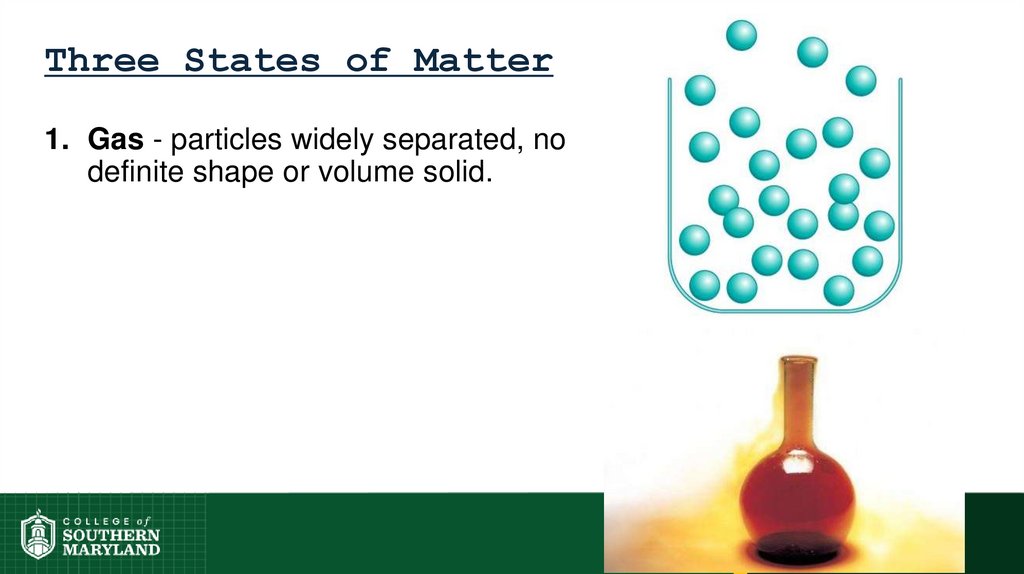
26. Three States of Matter
1. Gas - particles widely separated, nodefinite shape or volume solid.
27. Three States of Matter
1. Gas - particles widely separated, nodefinite shape or volume solid.
2. Liquid - particles closer together,
definite volume but no definite
shape.
28. Three States of Matter
1. Gas - particles widely separated, nodefinite shape or volume solid.
2. Liquid - particles closer together,
definite volume but no definite
shape.
3. Solid - particles are very close
together, define shape and definite
volume.
29.
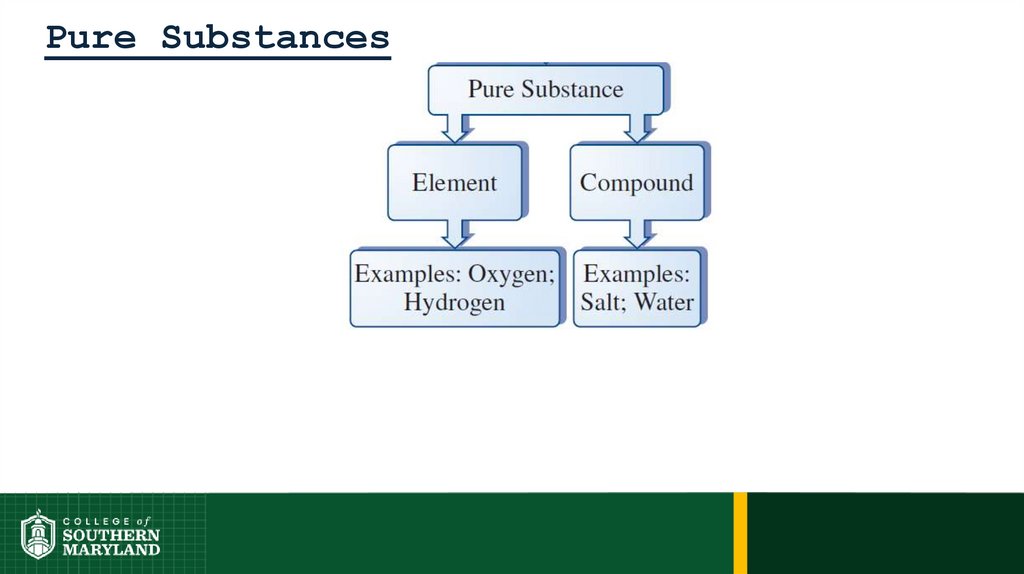
Composition of Matter30.
Composition of Matter• Pure substance - a substance that has only one component.
31.
Composition of Matter• Pure substance - a substance that has only one component.
• Mixture - a combination of two or more pure substances in which
each substance retains its own identity, not undergoing a chemical
reaction.
32.
Pure Substances33.
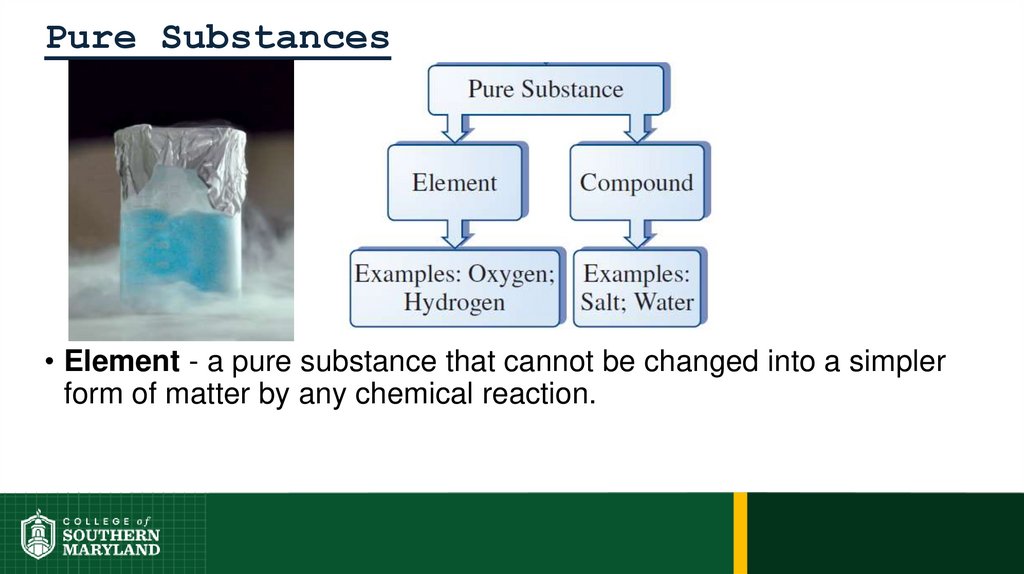
Pure Substances• Element - a pure substance that cannot be changed into a simpler
form of matter by any chemical reaction.
34.
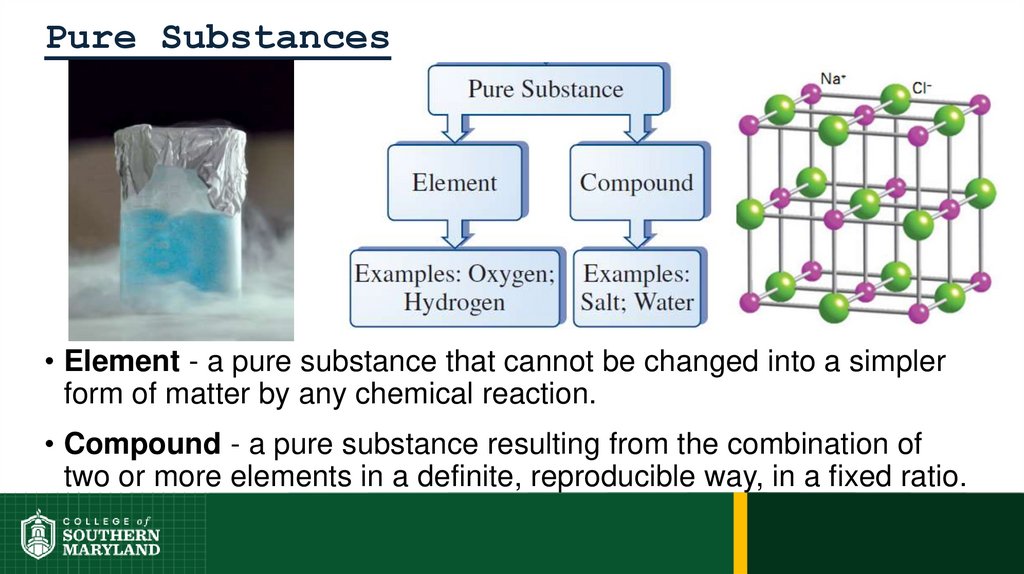
Pure Substances• Element - a pure substance that cannot be changed into a simpler
form of matter by any chemical reaction.
• Compound - a pure substance resulting from the combination of
two or more elements in a definite, reproducible way, in a fixed ratio.
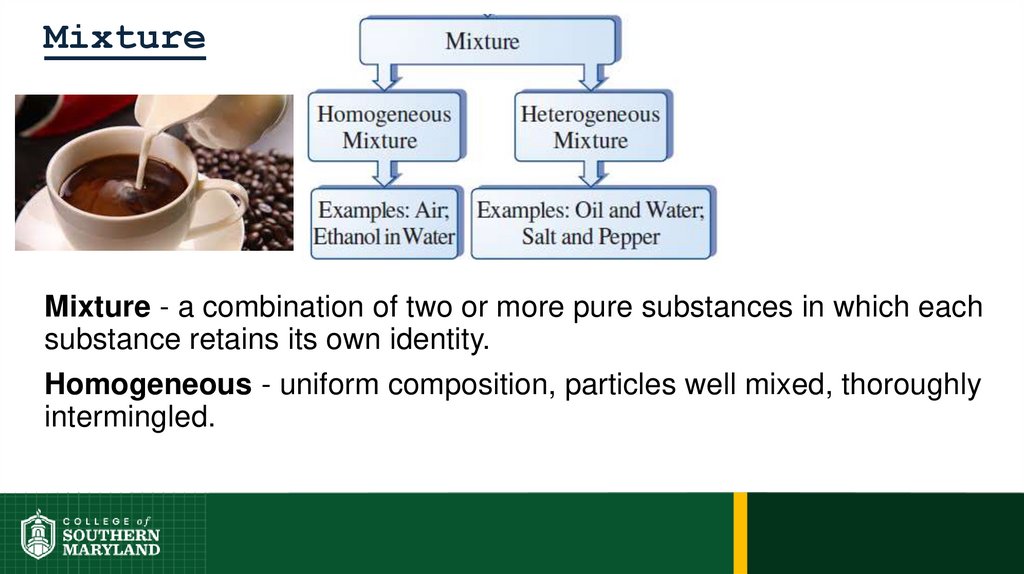
35. Mixture
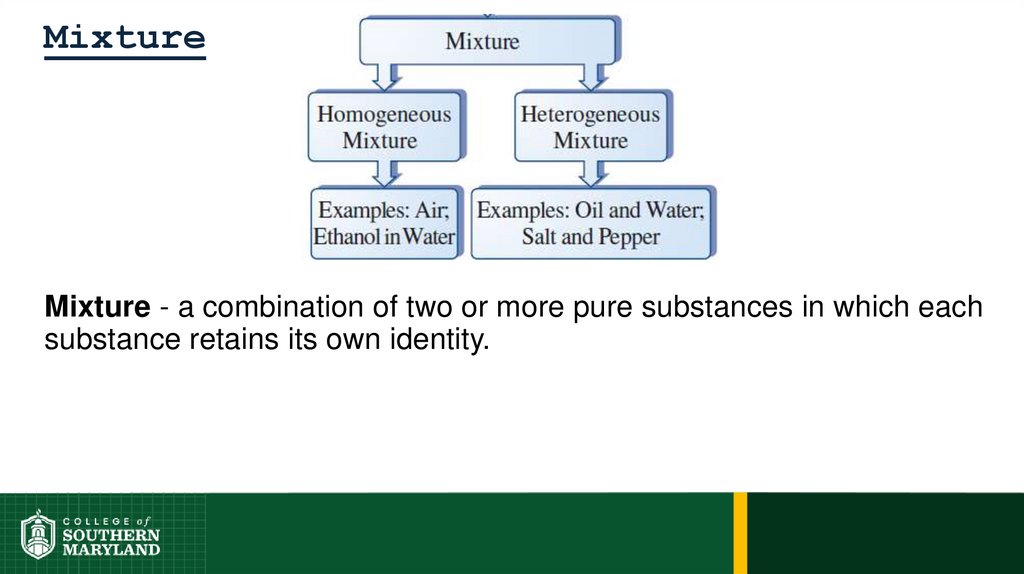
Mixture - a combination of two or more pure substances in which eachsubstance retains its own identity.
36. Mixture
Mixture - a combination of two or more pure substances in which eachsubstance retains its own identity.
Homogeneous - uniform composition, particles well mixed, thoroughly
intermingled.
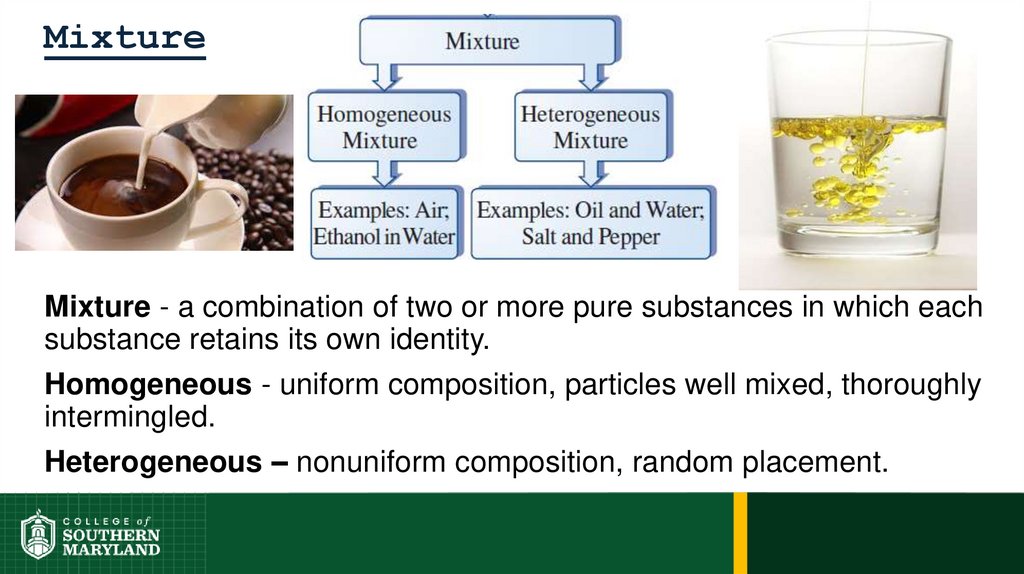
37. Mixture
Mixture - a combination of two or more pure substances in which eachsubstance retains its own identity.
Homogeneous - uniform composition, particles well mixed, thoroughly
intermingled.
Heterogeneous – nonuniform composition, random placement.
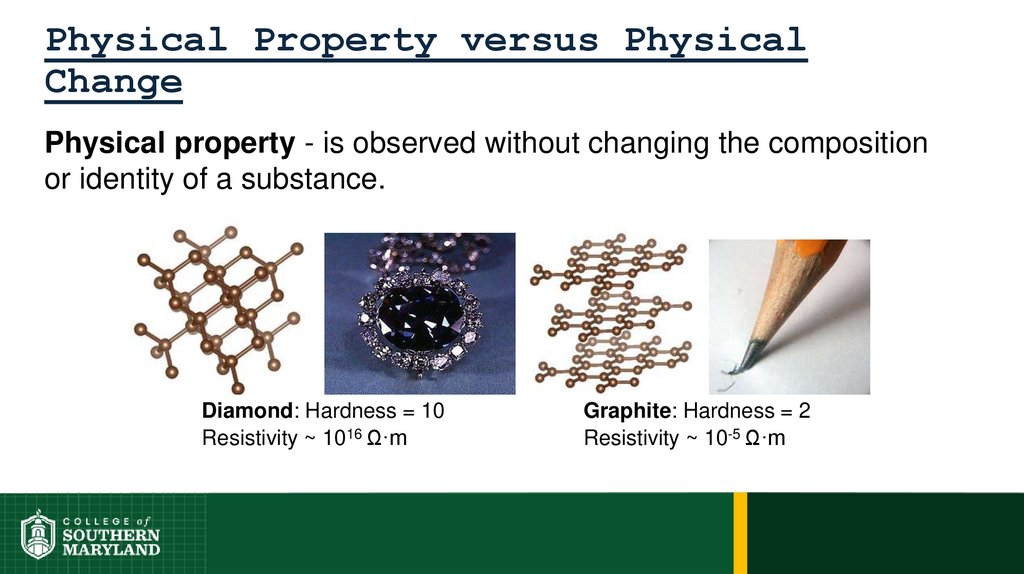
38. Physical Property versus Physical Change
Physical property - is observed without changing the compositionor identity of a substance.
Diamond: Hardness = 10
Resistivity ~ 1016 Ω·m
Graphite: Hardness = 2
Resistivity ~ 10-5 Ω·m
39. Physical Property versus Physical Change
Physical property - is observed without changing the compositionor identity of a substance.
Physical change - produces a recognizable difference in the
appearance of a substance without causing any change in its
composition or identity.
• conversion from one physical state to another.
• melting an ice cube.
40. Physical Property versus Physical Change
Physical property - is observed without changing the compositionor identity of a substance.
Physical change - produces a recognizable difference in the
appearance of a substance without causing any change in its
composition or identity.
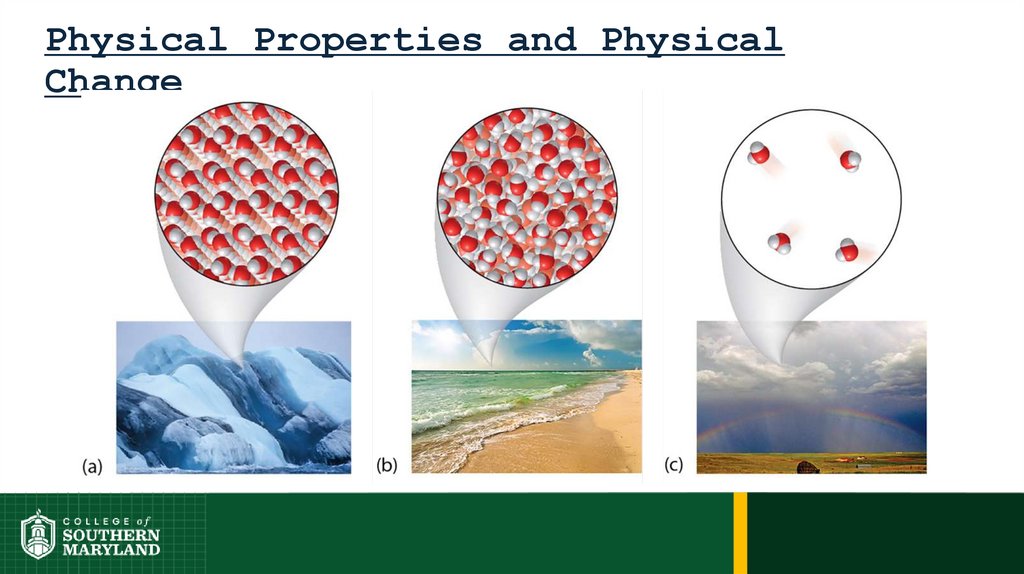
41.
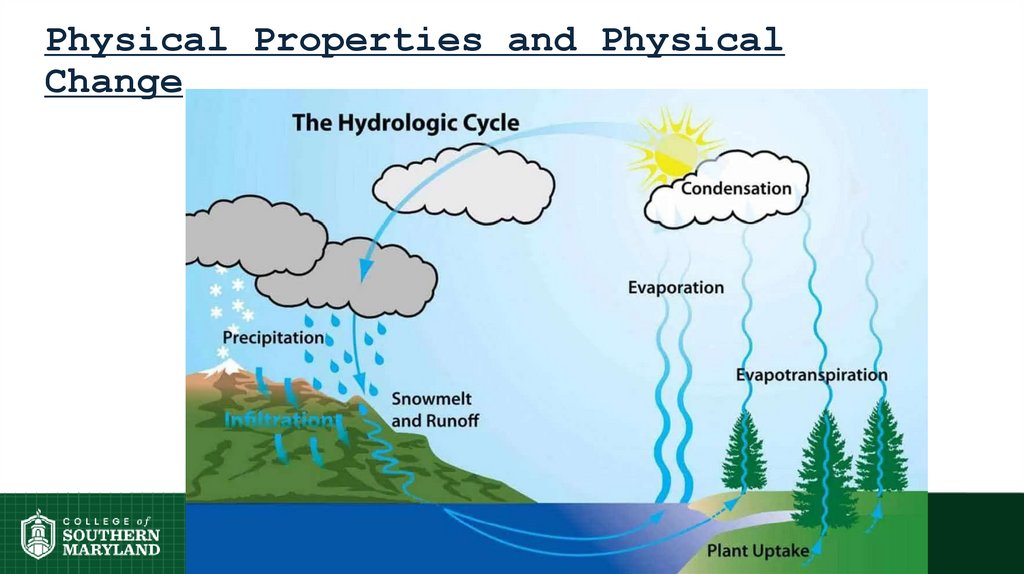
Physical Properties and PhysicalChange
42.
Physical Properties and PhysicalChange
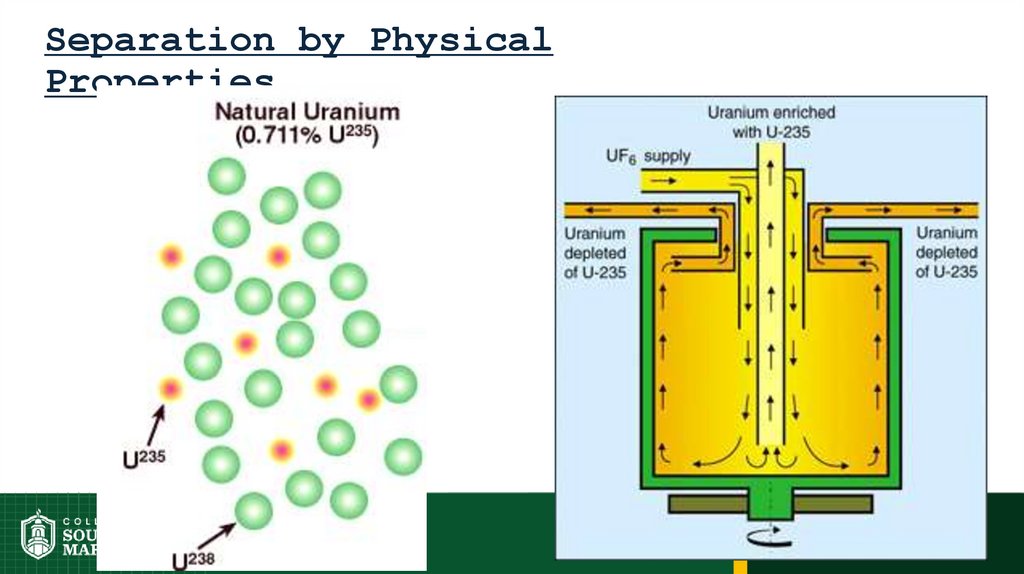
43. Separation by Physical Properties
Magnetic iron is separated from other nonmagnetic substances. Thisproperty is used as a large-scale process in the recycling industry.
44. Separation by Physical Properties
45.
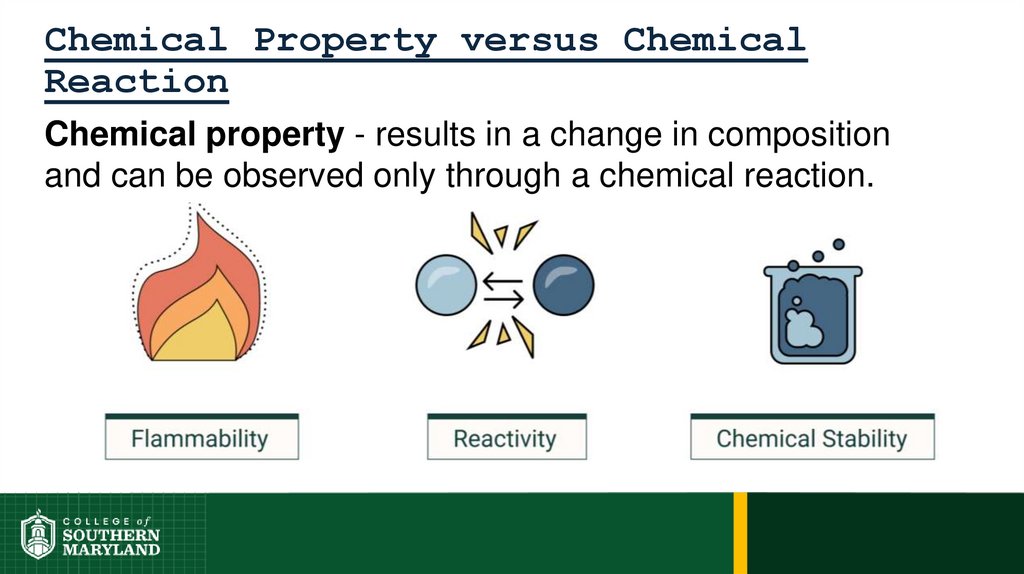
Chemical Property versus ChemicalReaction
Chemical property - results in a change in composition
and can be observed only through a chemical reaction.
46.
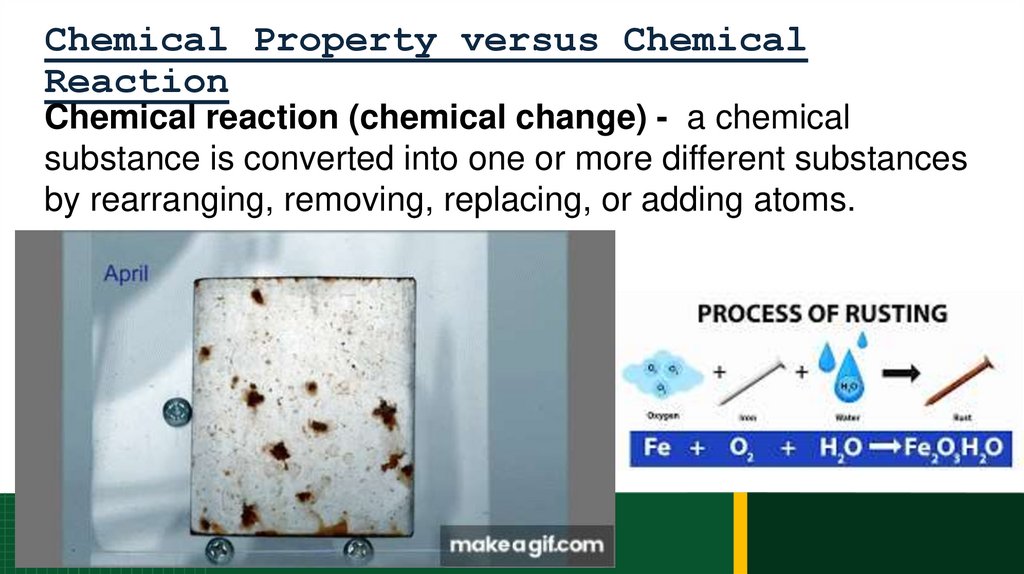
Chemical Property versus ChemicalReaction
Chemical reaction (chemical change) - a chemical
substance is converted into one or more different substances
by rearranging, removing, replacing, or adding atoms.
47. Classification of Properties
Classify the following as either a chemical orphysical property:
a. Color
Sulfur
Bismuth
Bromine
48. Classification of Properties
Classify the following as either a chemical orphysical property:
a. Color
b. Flammability
49. Classification of Properties
Classify the following as either a chemical orphysical property:
a. Color
b. Flammability
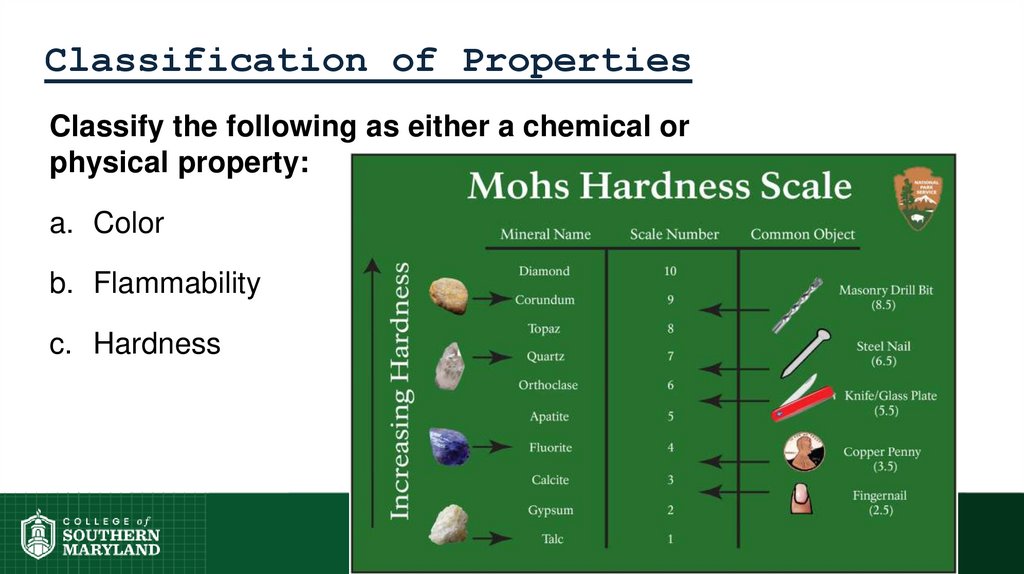
c. Hardness
50. Classification of Properties
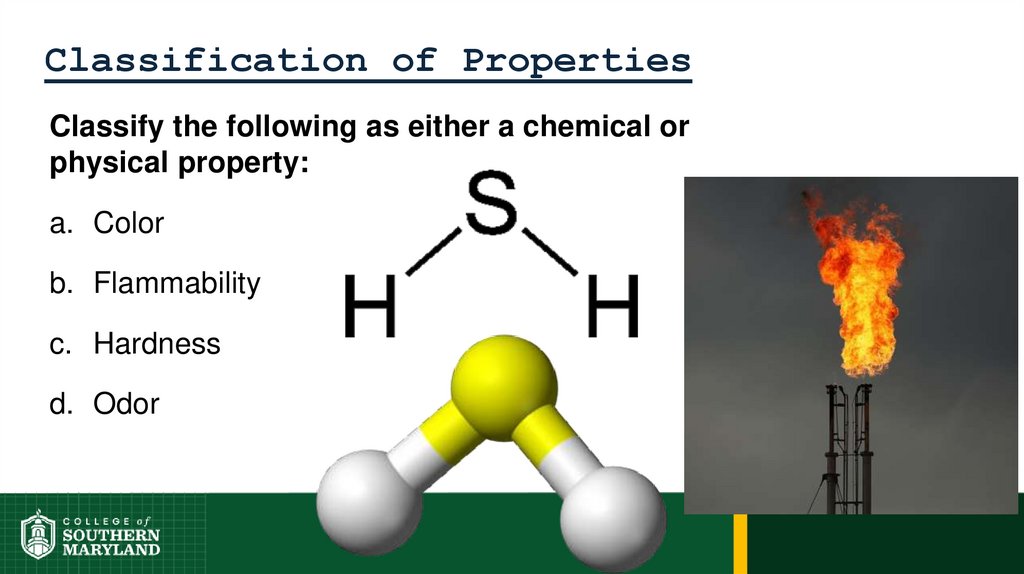
Classify the following as either a chemical orphysical property:
a. Color
b. Flammability
c. Hardness
d. Odor
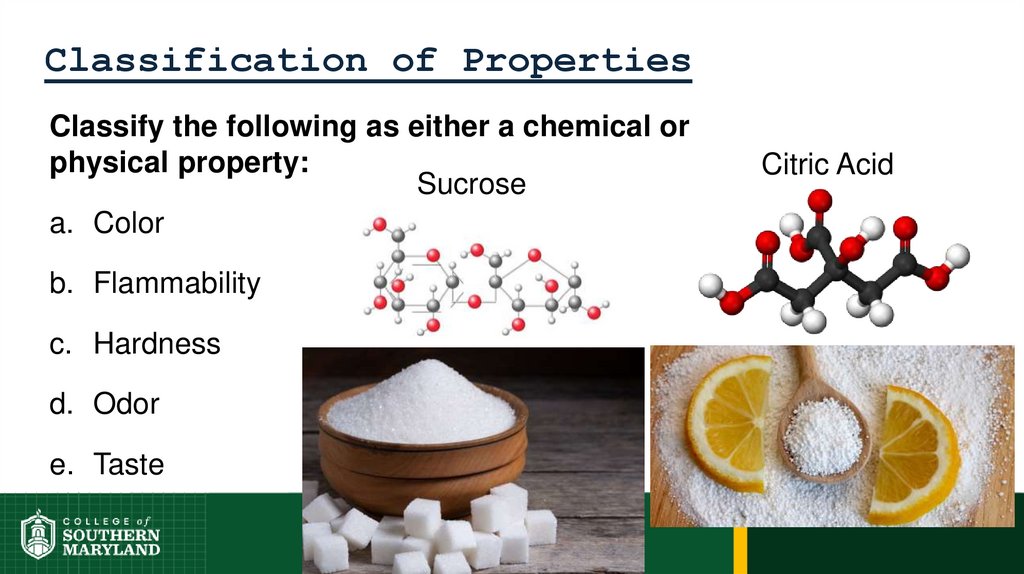
51. Classification of Properties
Classify the following as either a chemical orphysical property:
Sucrose
a. Color
b. Flammability
c. Hardness
d. Odor
e. Taste
Citric Acid
52.
Classification of ChangesClassify the following as either a chemical or
physical change:
a. Boiling water becomes steam.
53.
Classification of ChangesClassify the following as either a chemical or
physical change:
a. Boiling water becomes steam.
b. Butter turns rancid.
54.
Classification of ChangesClassify the following as either a chemical or
physical change:
a. Boiling water becomes steam.
b. Butter turns rancid.
c. Burning of wood.
55.
Classification of ChangesClassify the following as either a chemical or
physical change:
a. Boiling water becomes steam.
b. Butter turns rancid.
c. Burning of wood.
d. Mountain snow melting in spring.
56.
Classification of ChangesClassify the following as either a chemical or
physical change:
a. Boiling water becomes steam.
b. Butter turns rancid.
c. Burning of wood.
d. Mountain snow melting in spring.
e. Decay of leaves in winter.
57. Intensive and Extensive Properties
Intensive properties - a property of matter thatis independent of the quantity of the substance.
• Color.
58. Intensive and Extensive Properties
Intensive properties - a property of matter thatis independent of the quantity of the substance.
• Color.
• Melting Point.
59. Intensive and Extensive Properties
Extensive properties - a property of matter thatdepends on the quantity of the substance.
• Mass.
• Volume.
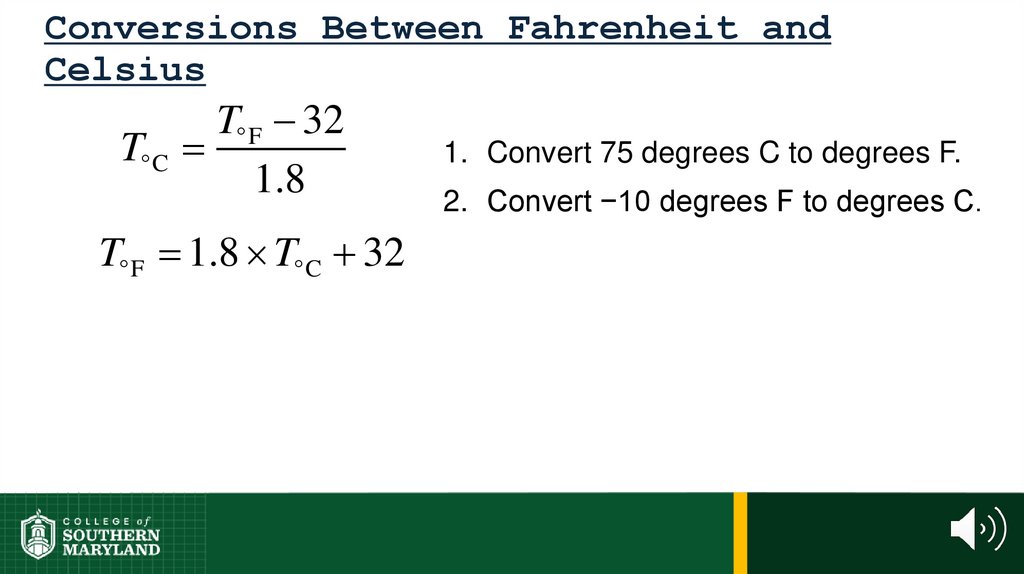
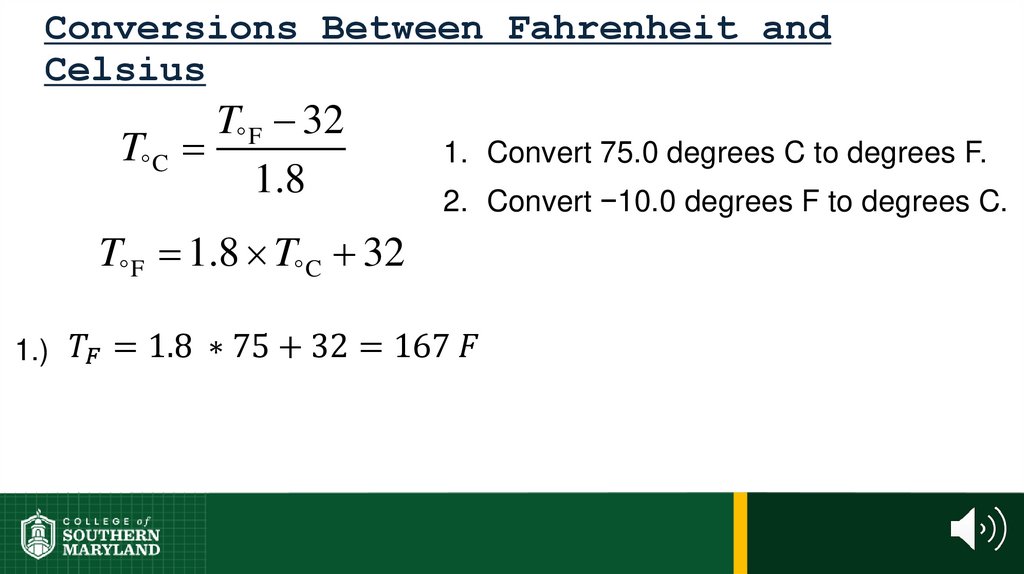
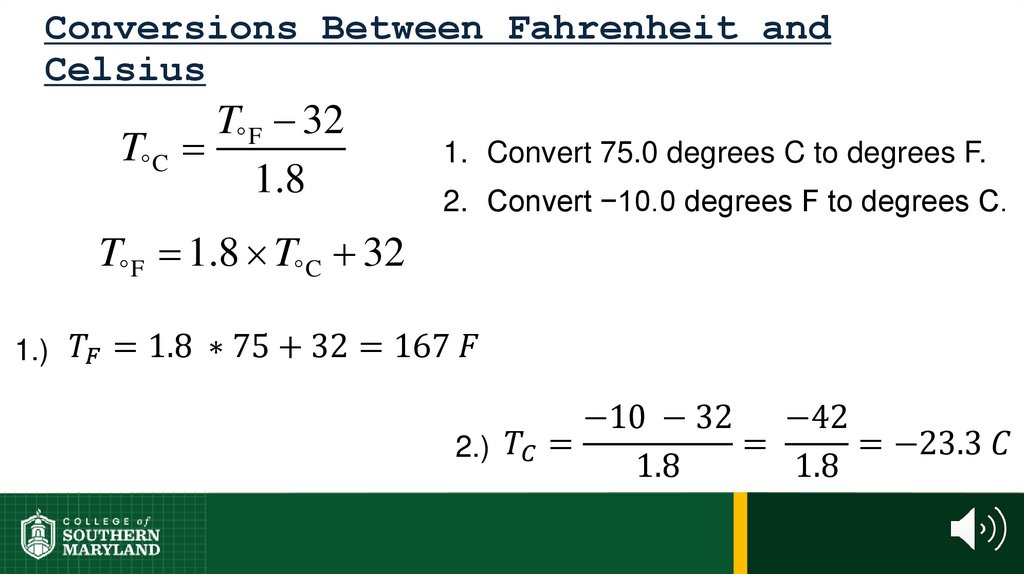
60. 1.4 The Units of Measurement
Units - the basic quantity of mass, volume or whatever quantity isbeing measured.
• A measurement is useless without its units.
61. 1.4 The Units of Measurement
Units - the basic quantity of mass, volume or whatever quantity isbeing measured.
• A measurement is useless without its units.
English system - a collection of functionally unrelated units.
• Difficult to convert from one unit to another.
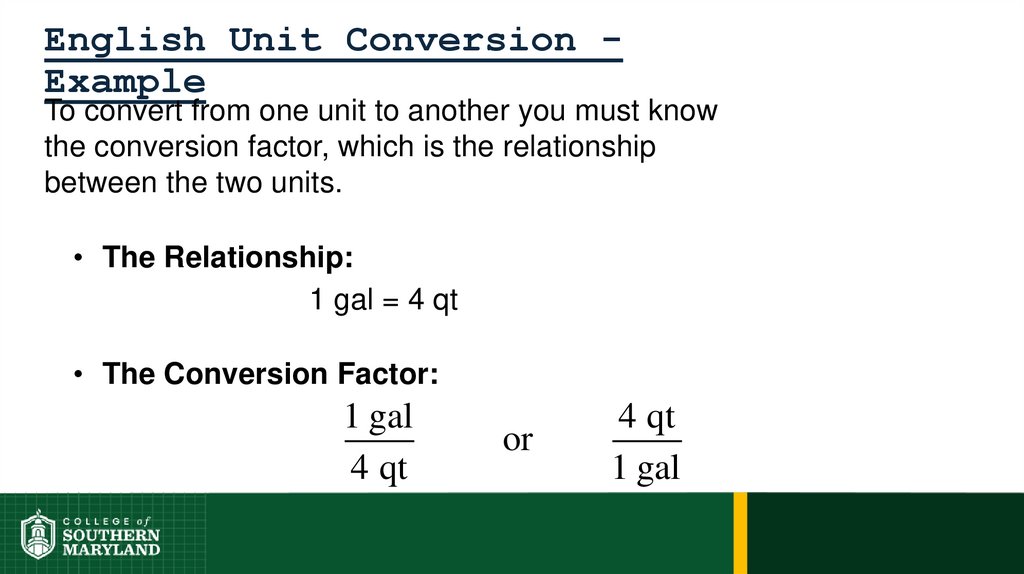
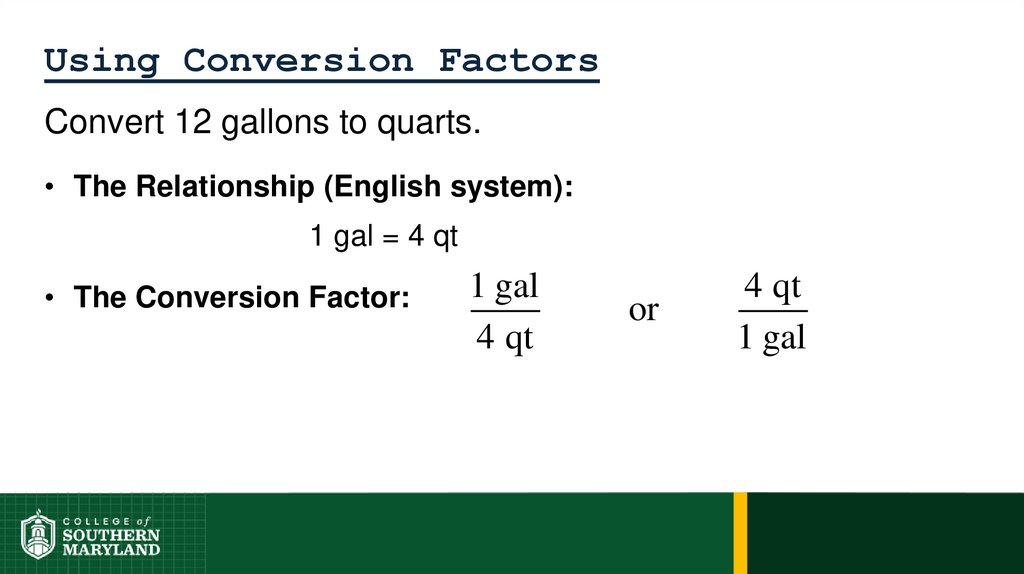
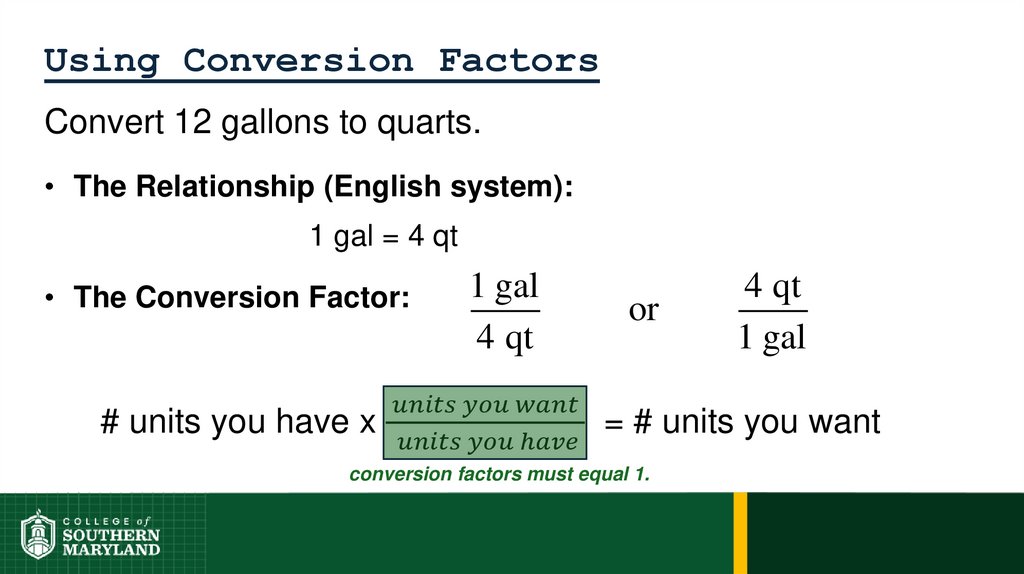
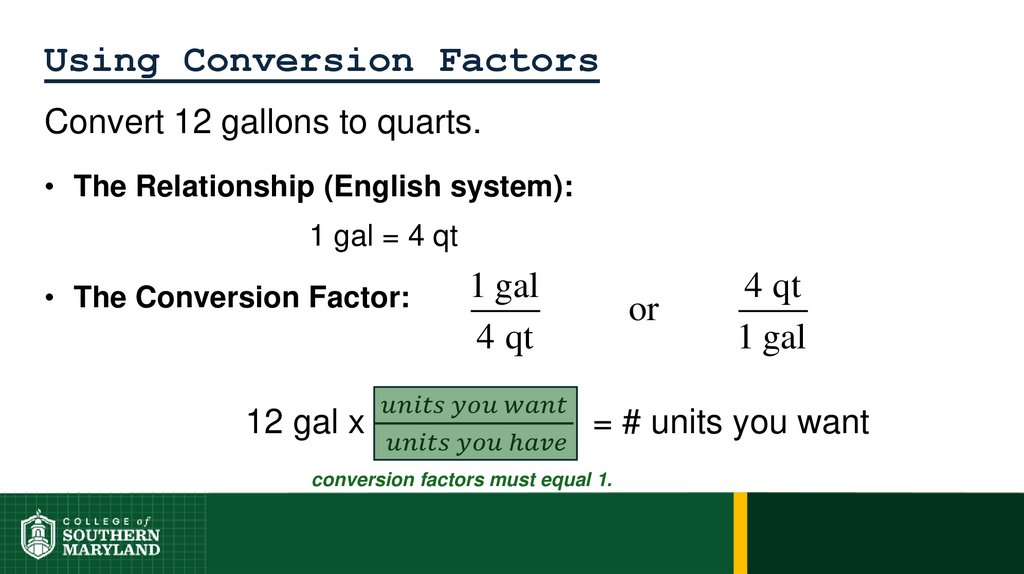
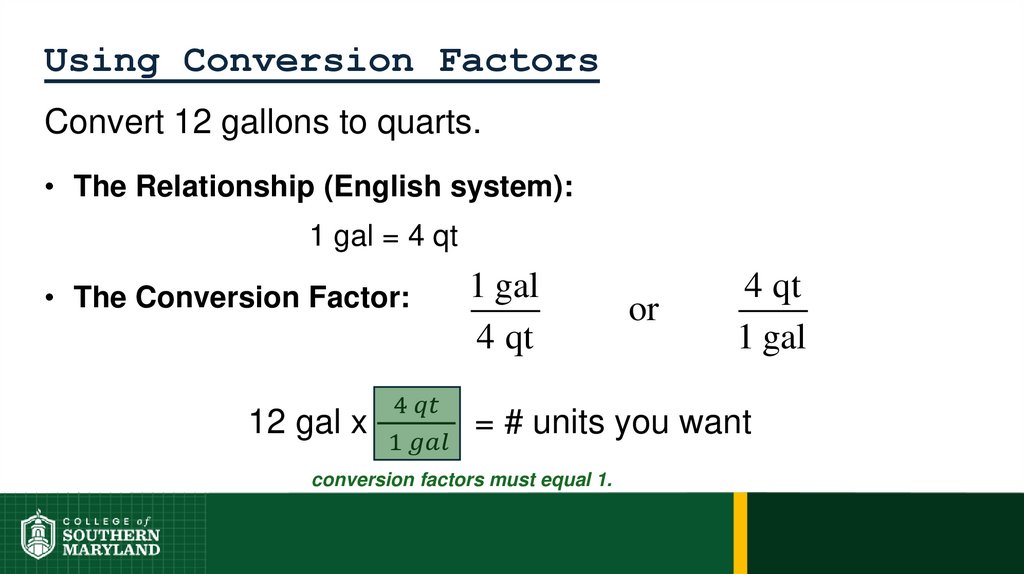
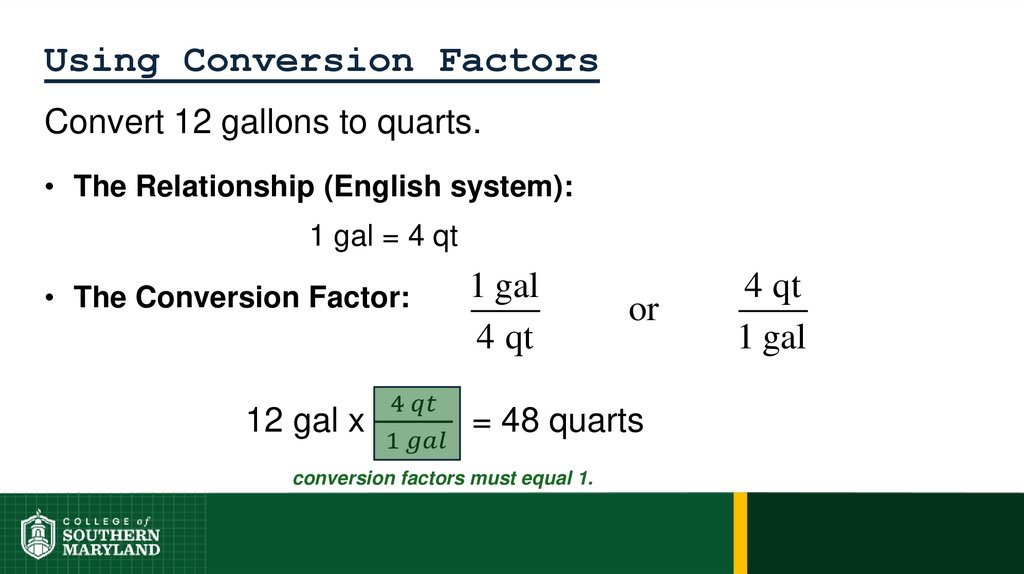
1 foot = 12 inches = 0.33 yard = 1/5280 miles
62. 1.4 The Units of Measurement
Units - the basic quantity of mass, volume or whatever quantity isbeing measured.
• A measurement is useless without its units.
English system - a collection of functionally unrelated units.
• Difficult to convert from one unit to another.
1 foot = 12 inches = 0.33 yard = 1/5280 miles
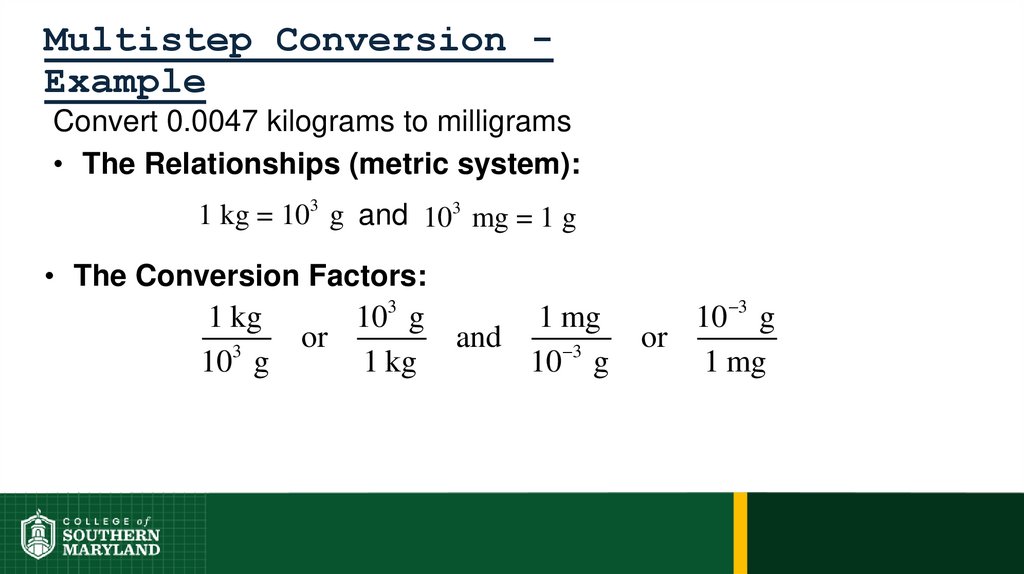
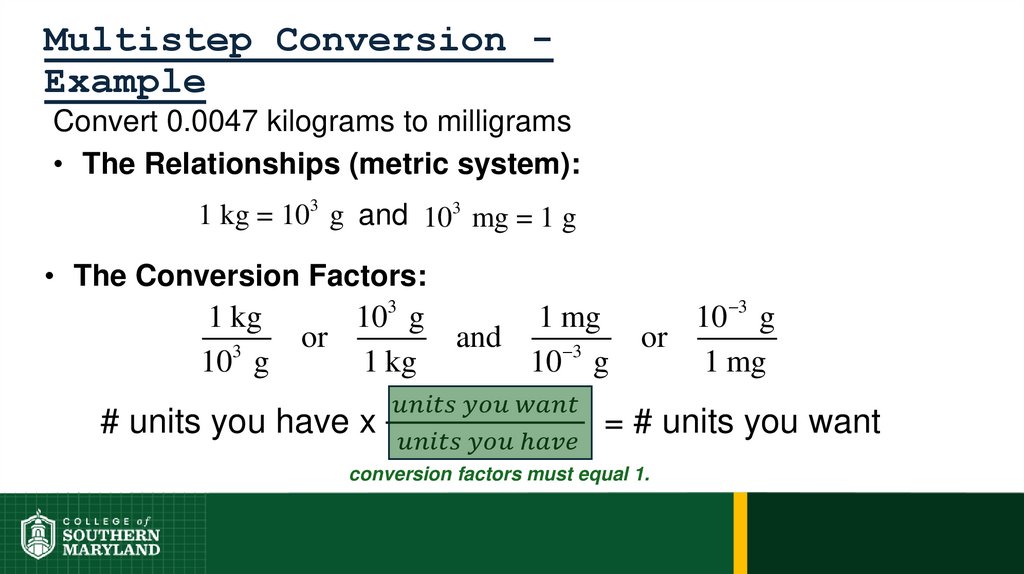
Metric System - composed of a set of units that are related to
each other decimally, systematic.
• Units relate by powers of tens.
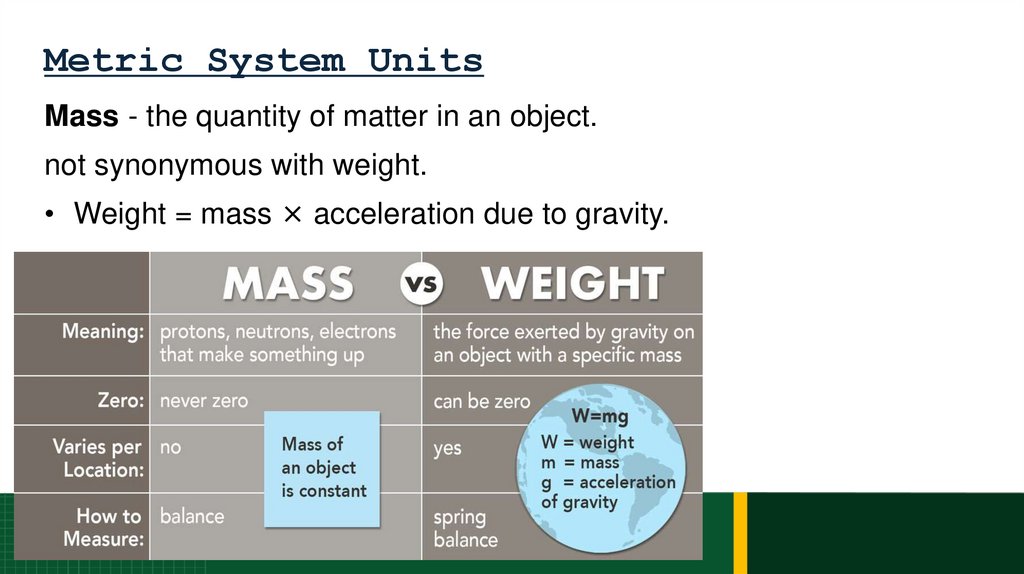
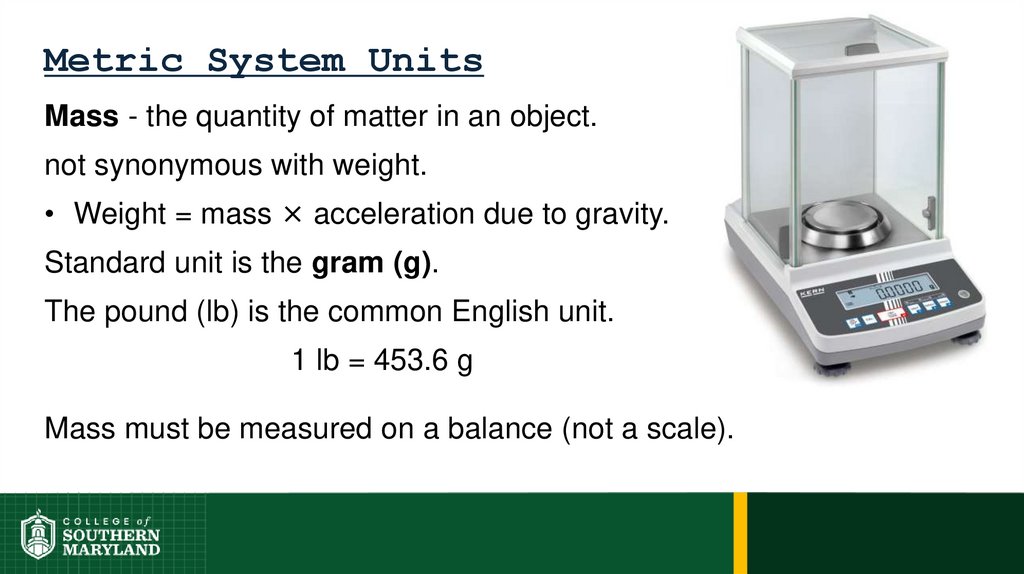
63. Metric System Units
Mass - the quantity of matter in an object.not synonymous with weight.
• Weight = mass × acceleration due to gravity.
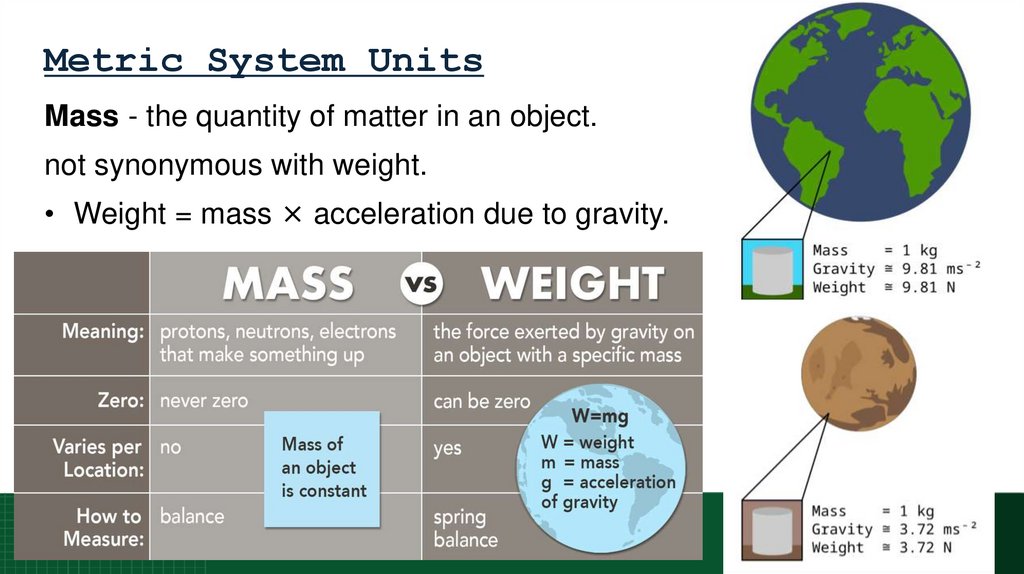
64. Metric System Units
Mass - the quantity of matter in an object.not synonymous with weight.
• Weight = mass × acceleration due to gravity.
65. Metric System Units
Mass - the quantity of matter in an object.not synonymous with weight.
• Weight = mass × acceleration due to gravity.
Standard unit is the gram (g).
The pound (lb) is the common English unit.
1 lb = 453.6 g
Mass must be measured on a balance (not a scale).
66. Metric System Units
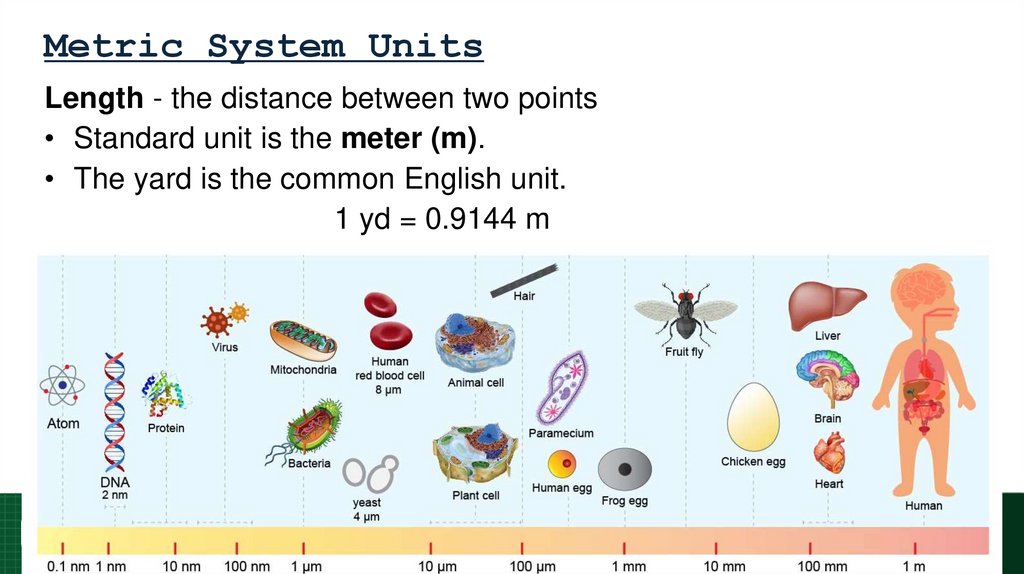
Length - the distance between two points• Standard unit is the meter (m).
• The yard is the common English unit.
1 yd = 0.9144 m
Volume - the space occupied by an object
• Standard unit is the liter (L).
• The quart is the common English unit.
1 qt = 0.9464 L
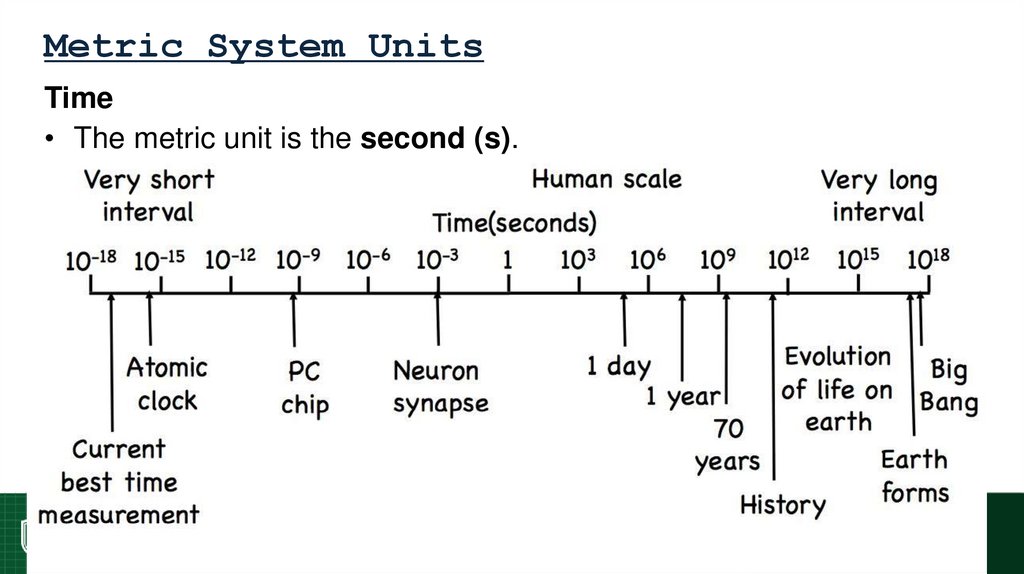
Time
• The metric unit is the second (s).
67. Metric System Units
Length - the distance between two points• Standard unit is the meter (m).
• The yard is the common English unit.
1 yd = 0.9144 m
68. Metric System Units
Length - the distance between two points• Standard unit is the meter (m).
• The yard is the common English unit.
1 yd = 0.9144 m
Volume - the space occupied by an object
• Standard unit is the liter (L).
• The quart is the common English unit.
1 qt = 0.9464 L
69. Metric System Units
Time• The metric unit is the second (s).
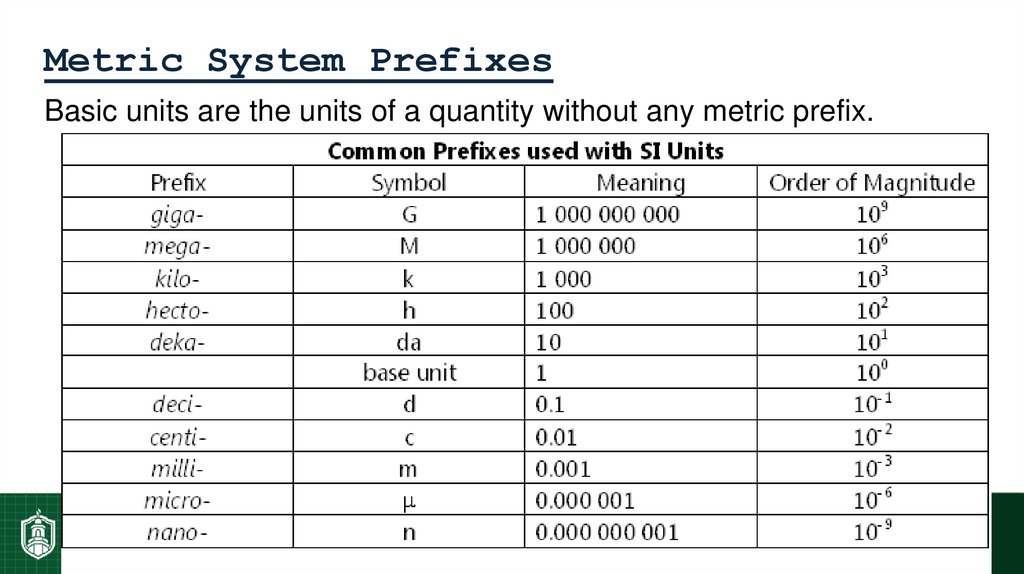
70. Metric System Prefixes
Basic units are the units of a quantity without any metric prefix.71. Relationship among various volume units
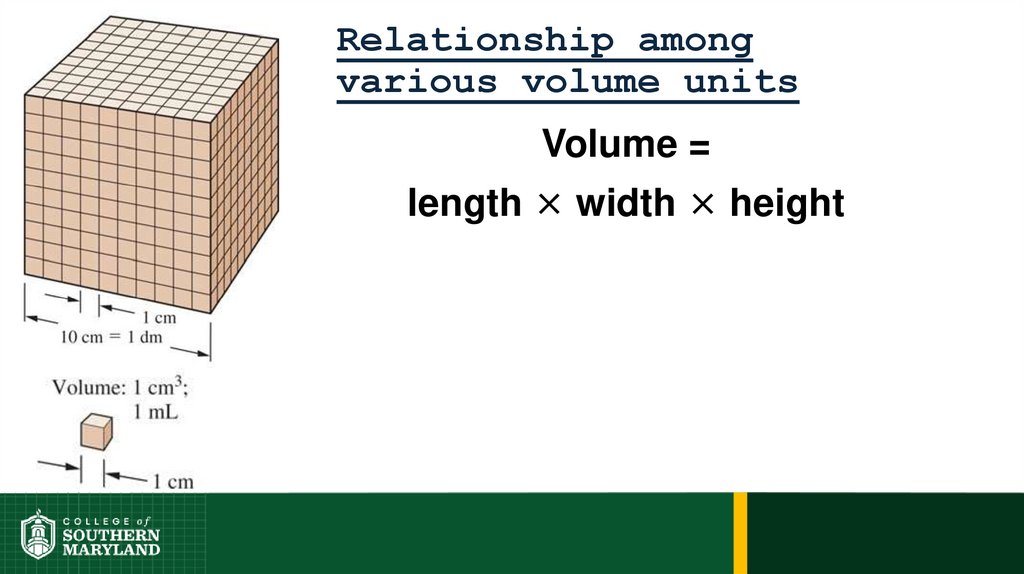
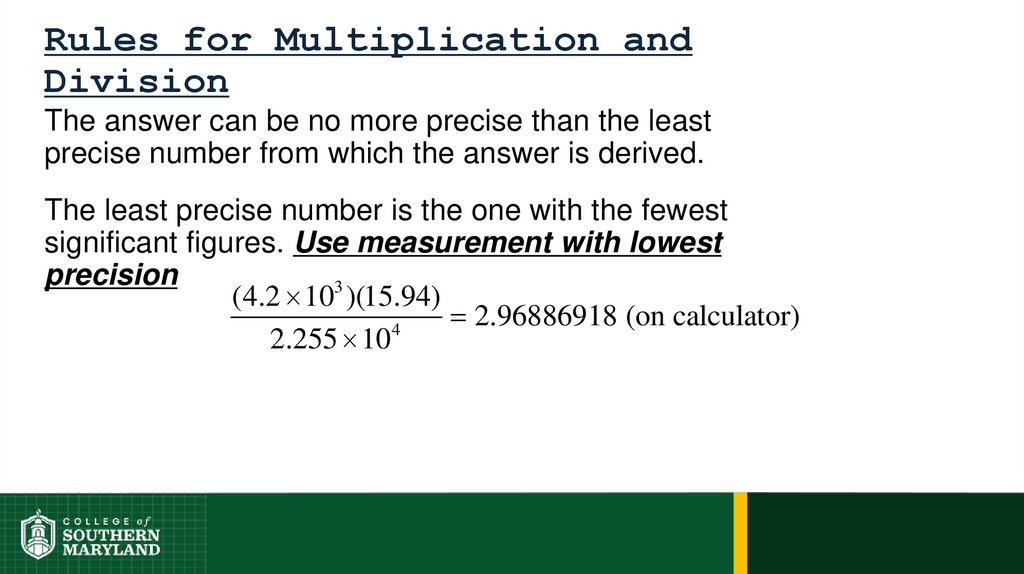
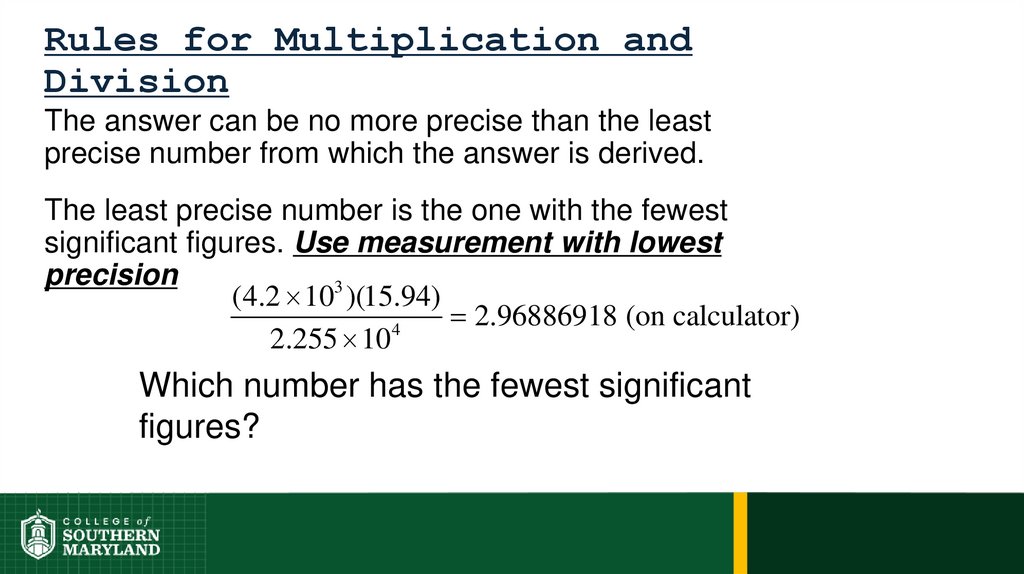
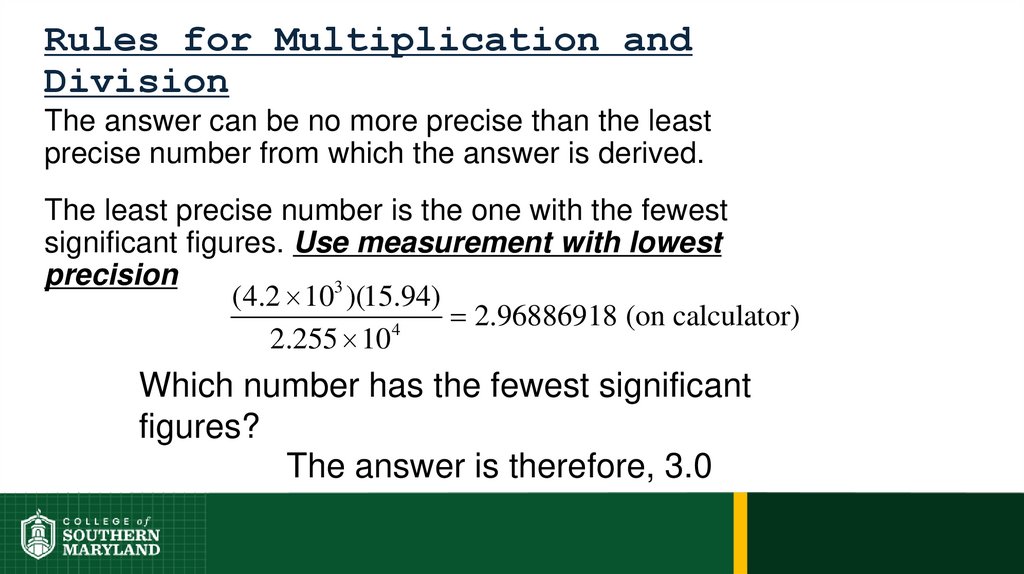
Volume =length × width × height
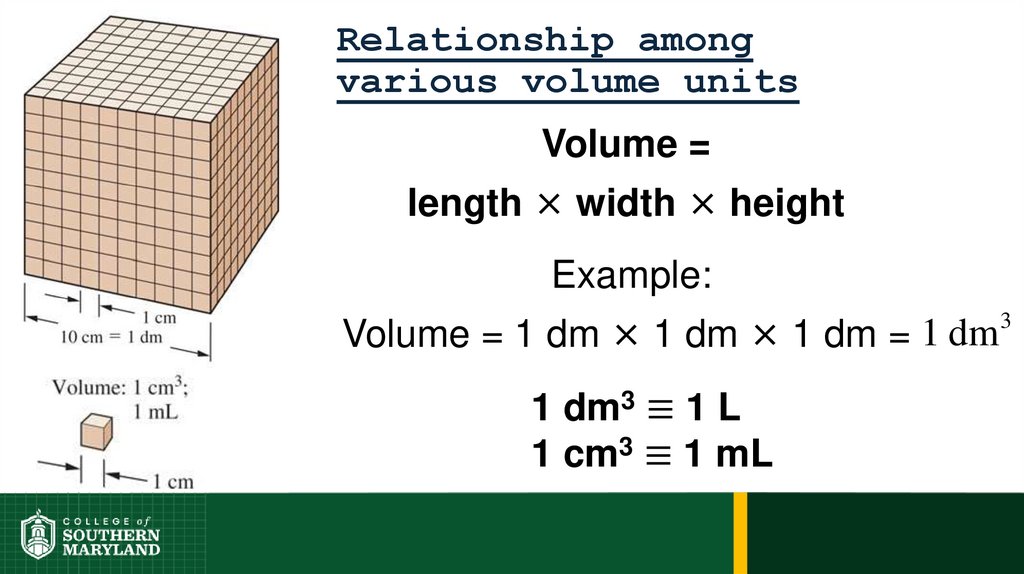
72. Relationship among various volume units
Volume =length × width × height
Example:
Volume = 1 dm × 1 dm × 1 dm = 1 dm
1 dm3 ≡ 1 L
1 cm3 ≡ 1 mL
3
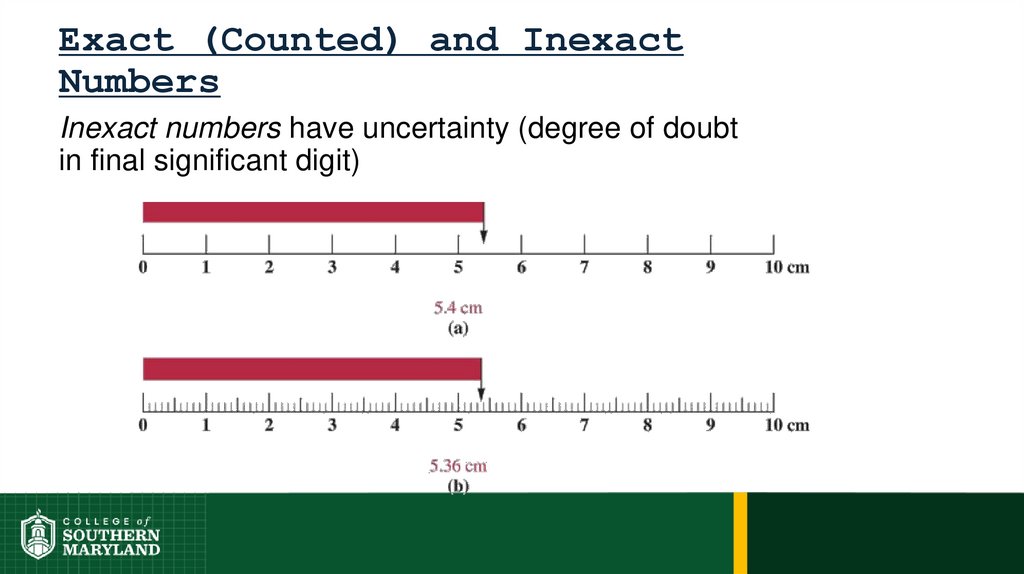
73. 1.5 The Numbers of Measurement
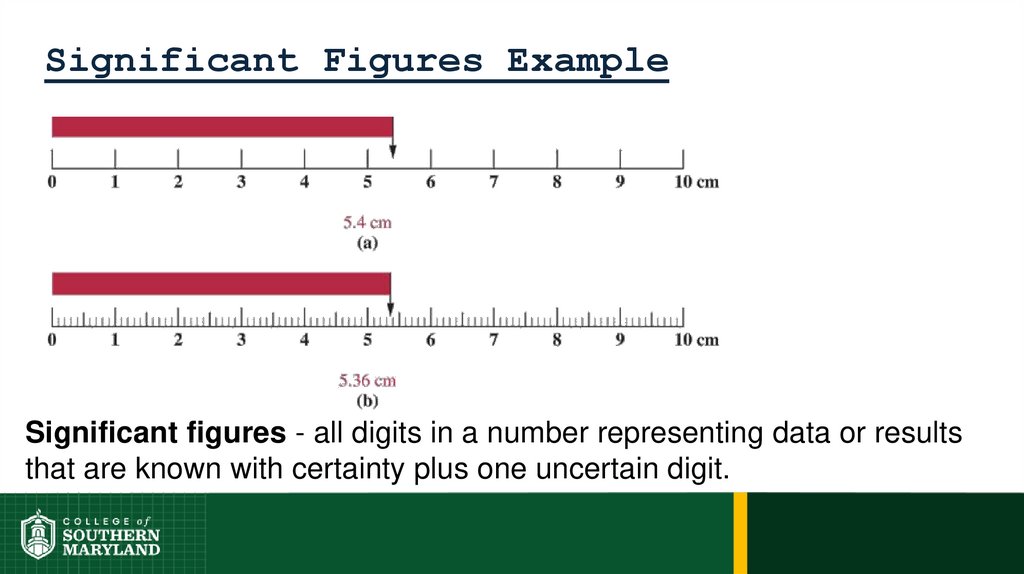
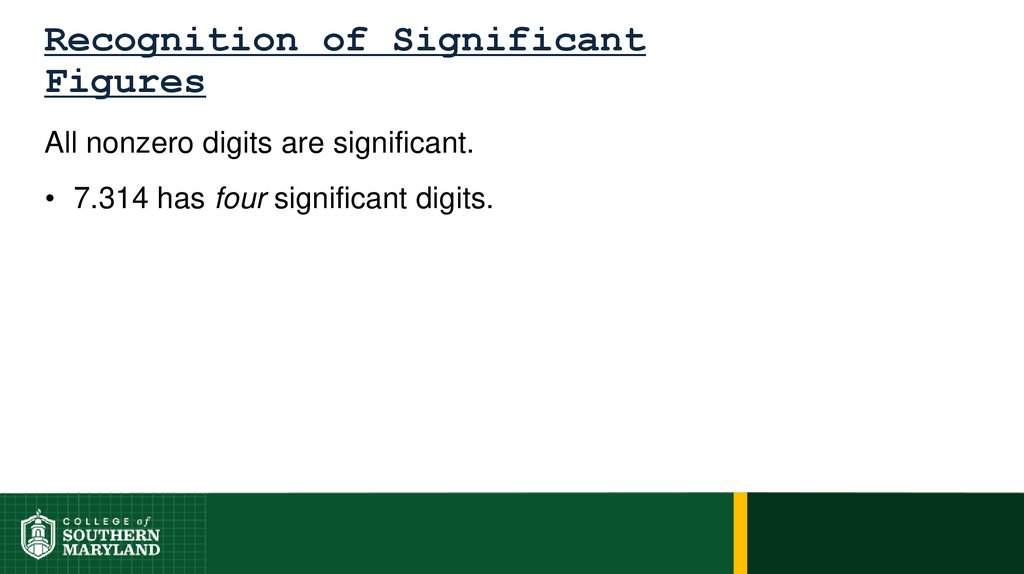
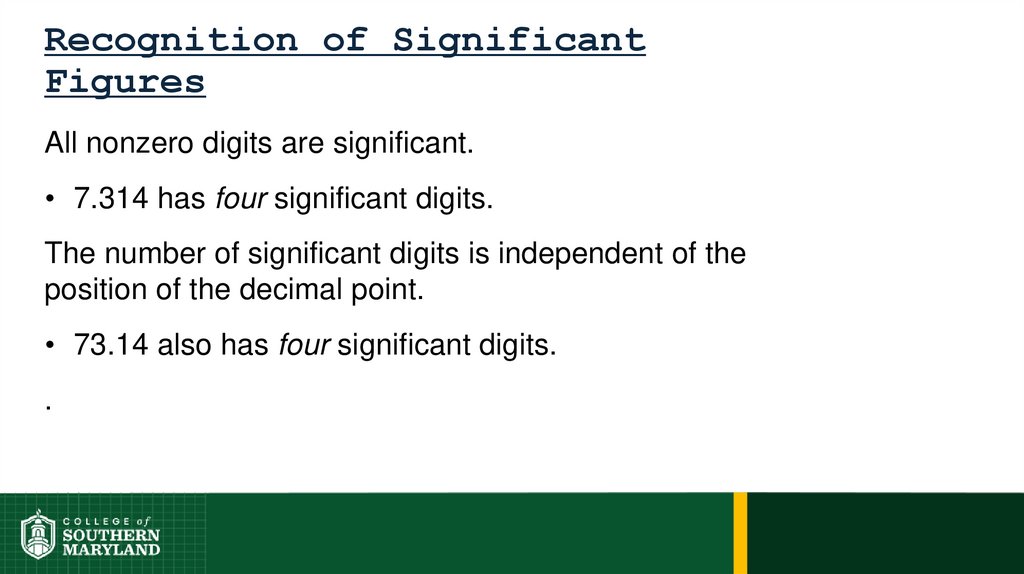
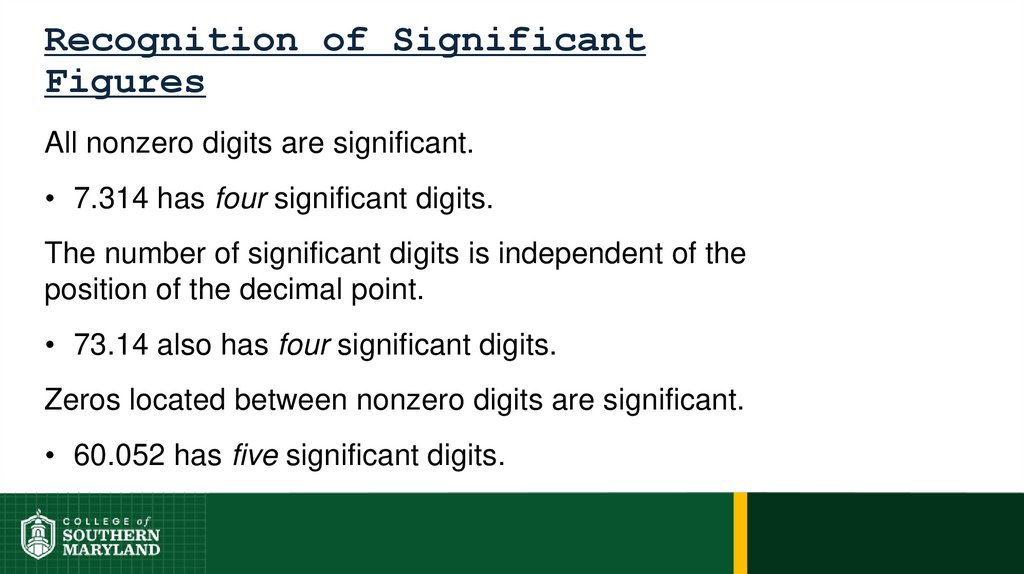
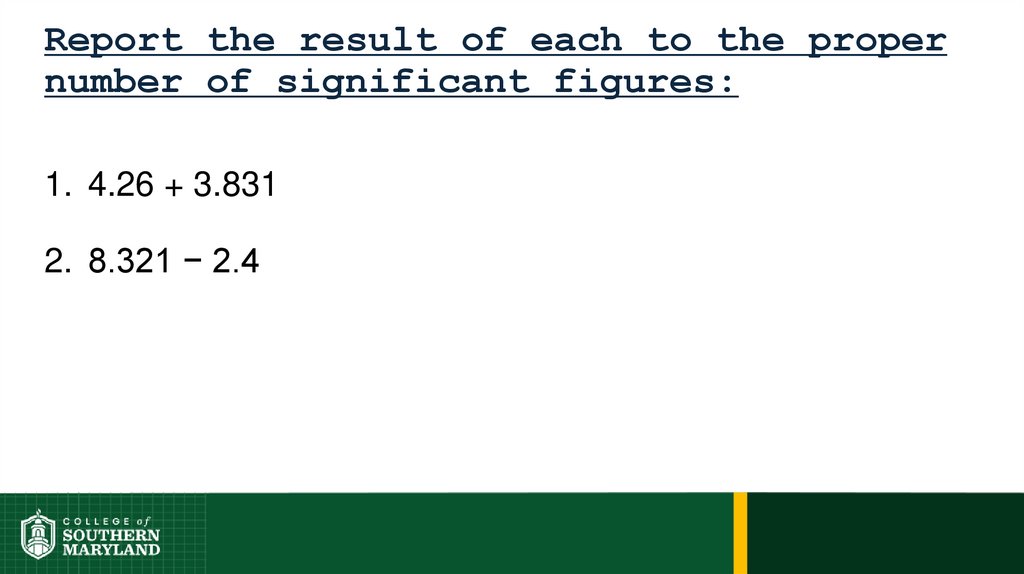
Information-bearing digits or figures in a number aresignificant figures.
74. 1.5 The Numbers of Measurement
Information-bearing digits or figures in a number aresignificant figures.
The measuring device used determines the number
of significant figures in a measurement.
The degree of uncertainty associated with a
measurement is indicated by the number of figures
used to represent the information.
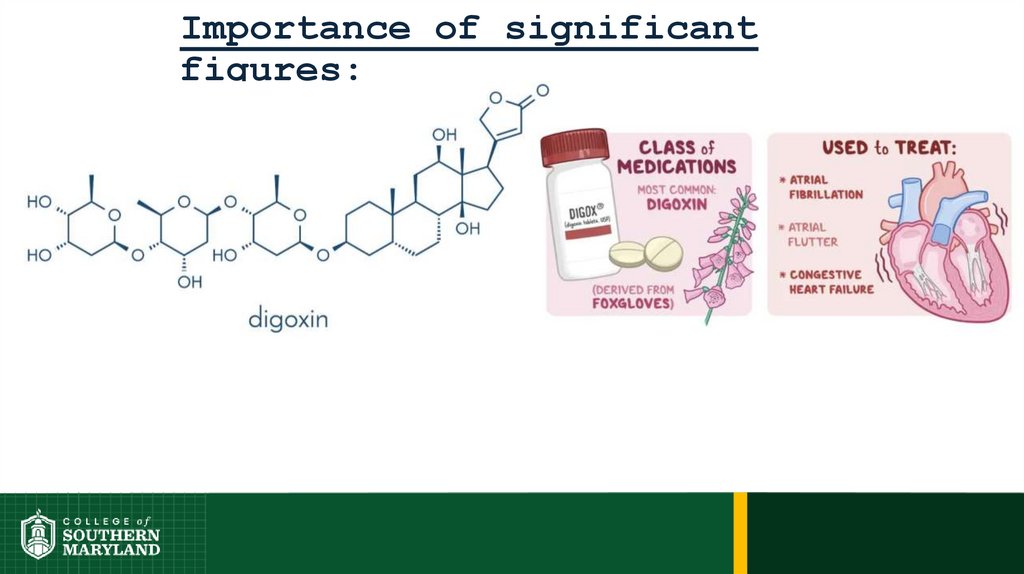
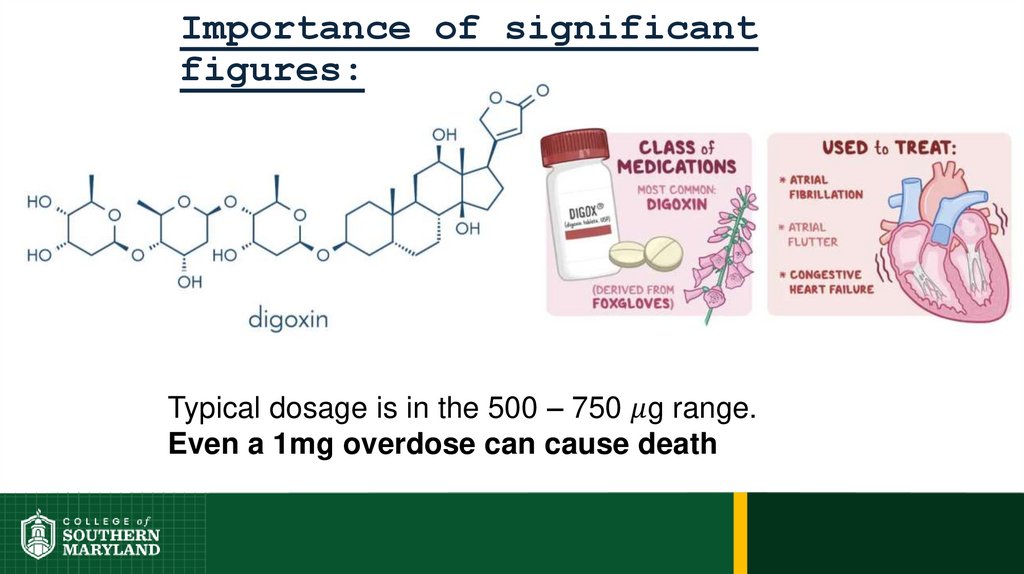
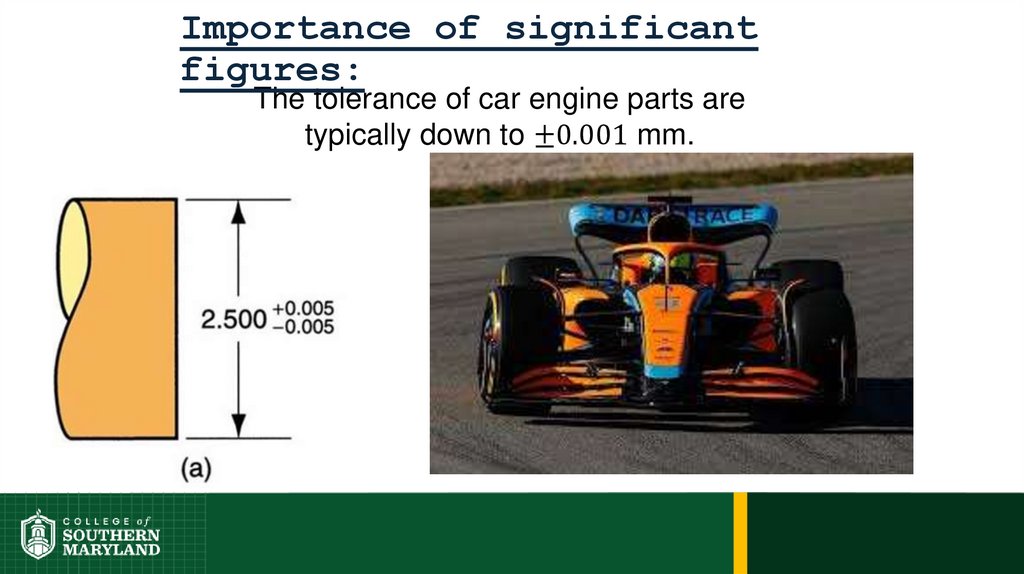
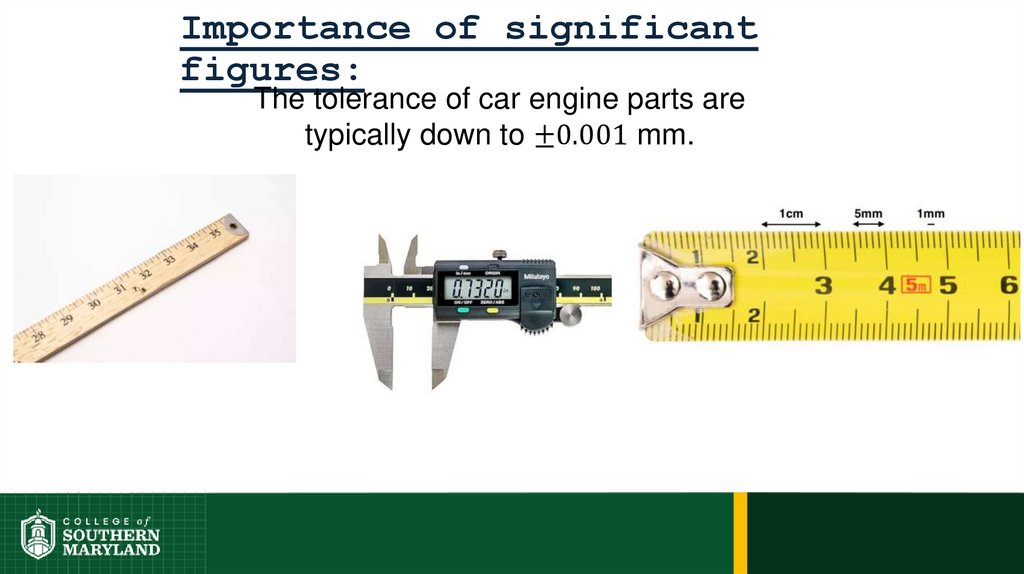
75. Importance of significant figures:
76.
Importance of significantfigures:
Typical dosage is in the 500 – 750