Similar presentations:
Chemical_Compounds__Chapter_3 (2)
1.
GENERAL CHEMISTRYPrinciples and Modern Applications
PETRUCCI
HERRING
MADURA
Chemical
Compounds
PHILIP DUTTON
UNIVERSITY OF WINDSOR
DEPARTMENT OF CHEMISTRY AND
BIOCHEMISTRY
TENTH EDITION
BISSONNETTE
3
2. Chemical Compounds
CONTENTS3-1
Types of Chemical
Compounds and Their
Formulas
3-2
The Mole Concept and
Chemical Compounds
3-3
Composition of Chemical
Compounds
3-4
Oxidation States: A Useful
Tool in Describing Chemical
Compounds
3-5
Naming Compounds: Organic
and Inorganic Compounds
3-6
Names and Formulas of
Inorganic Compounds
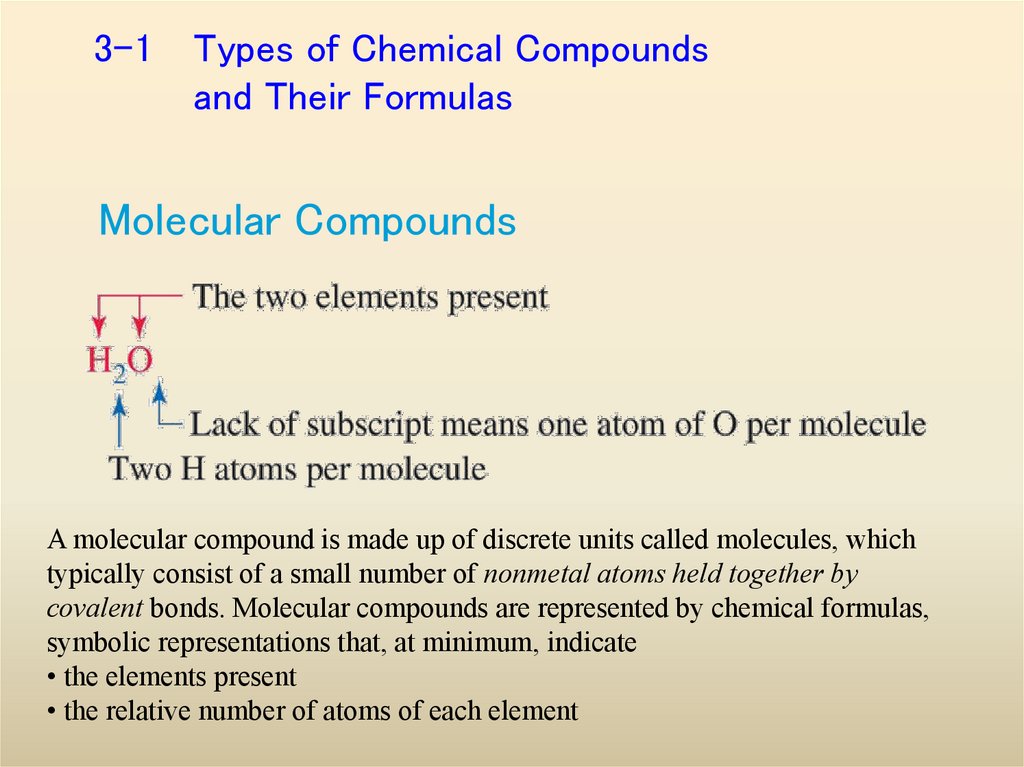
3. 3-1 Types of Chemical Compounds and Their Formulas
Molecular CompoundsA molecular compound is made up of discrete units called molecules, which
typically consist of a small number of nonmetal atoms held together by
covalent bonds. Molecular compounds are represented by chemical formulas,
symbolic representations that, at minimum, indicate
• the elements present
• the relative number of atoms of each element
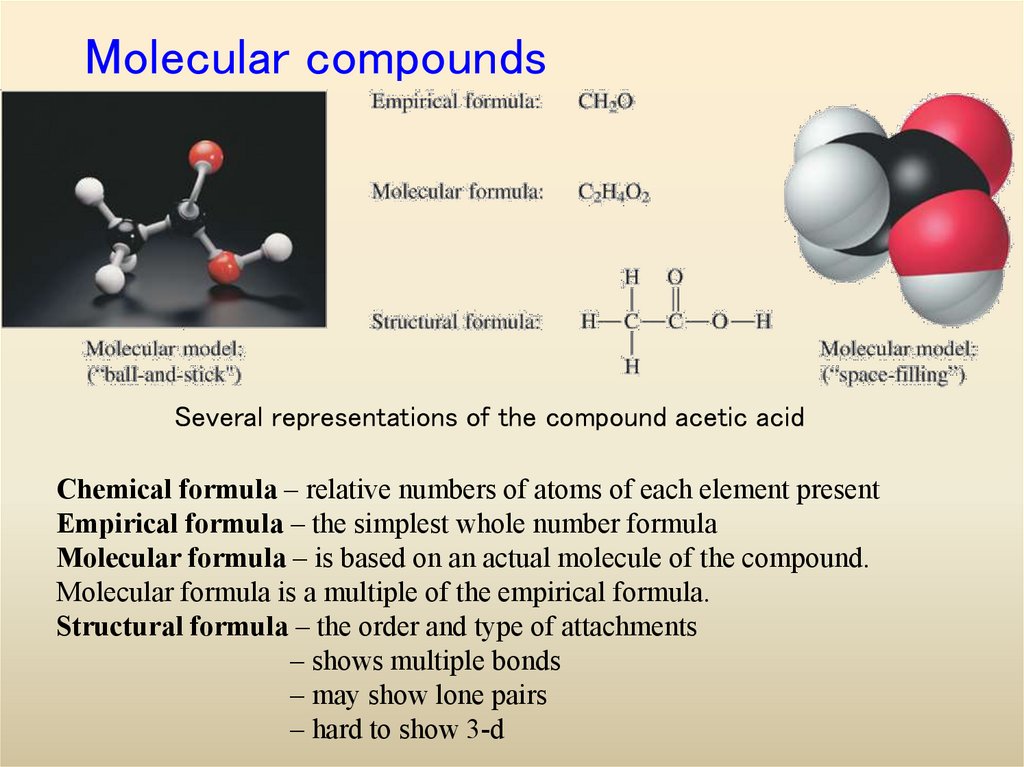
4. Molecular compounds
Several representations of the compound acetic acidChemical formula – relative numbers of atoms of each element present
Empirical formula – the simplest whole number formula
Molecular formula – is based on an actual molecule of the compound.
Molecular formula is a multiple of the empirical formula.
Structural formula – the order and type of attachments
– shows multiple bonds
– may show lone pairs
– hard to show 3-d
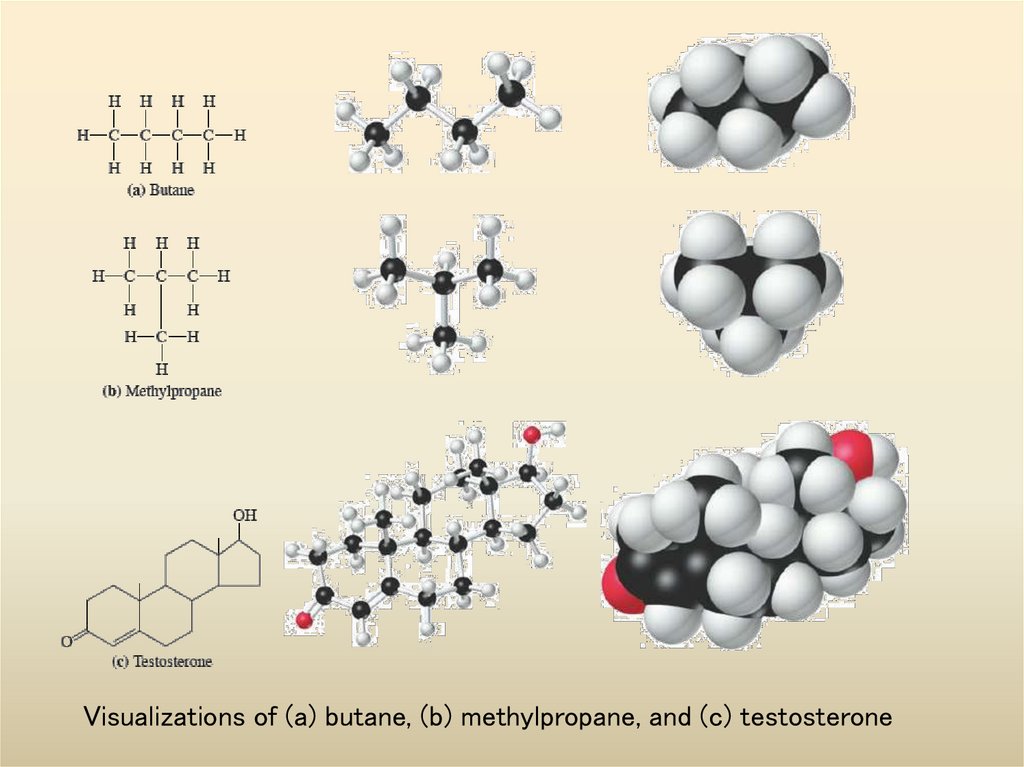
5. Visualizations of (a) butane, (b) methylpropane, and (c) testosterone
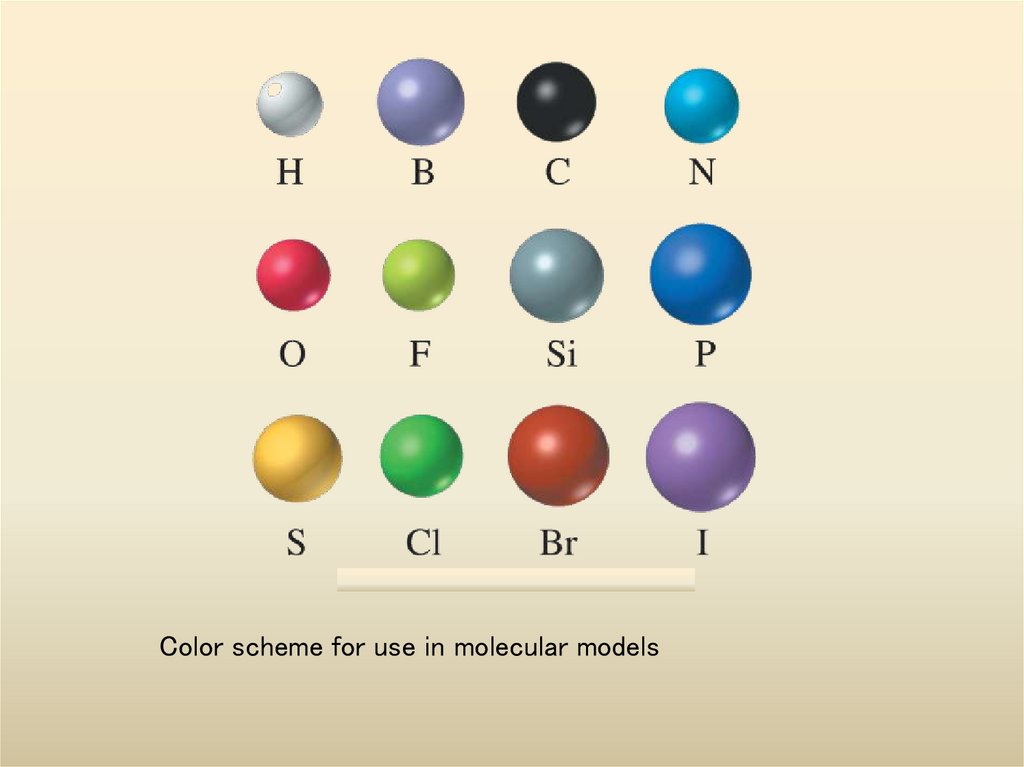
6. Color scheme for use in molecular models
7. Ionic Compounds
• Atoms of almost all elements can gain or lose electrons toform charged species called ions.
• Compounds composed of ions are known as ionic
compounds.
+ Metals tend to lose electrons to form positively
charged ions called cations.
- Non-metals tend to gain electrons to form negatively
charged ions called anions.
Positive and negative ions joined together by electrostatic
forces. Metals tend to lose electrons to form cations. Nonmetals tend to gain electrons to form anions. Ionic solids
formulae are reported as the formula unit – unordered to
call it a molecular formula
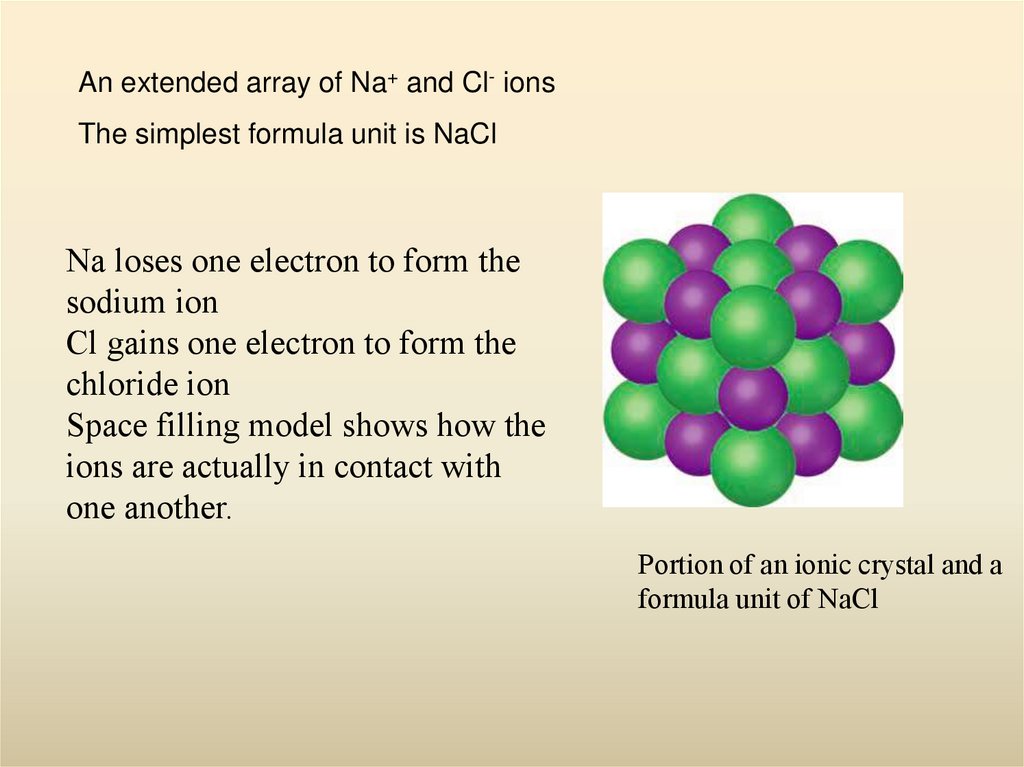
8. Portion of an ionic crystal and a formula unit of NaCl
An extended array of Na+ and Cl- ionsThe simplest formula unit is NaCl
Na loses one electron to form the
sodium ion
Cl gains one electron to form the
chloride ion
Space filling model shows how the
ions are actually in contact with
one another.
Portion of an ionic crystal and a
formula unit of NaCl
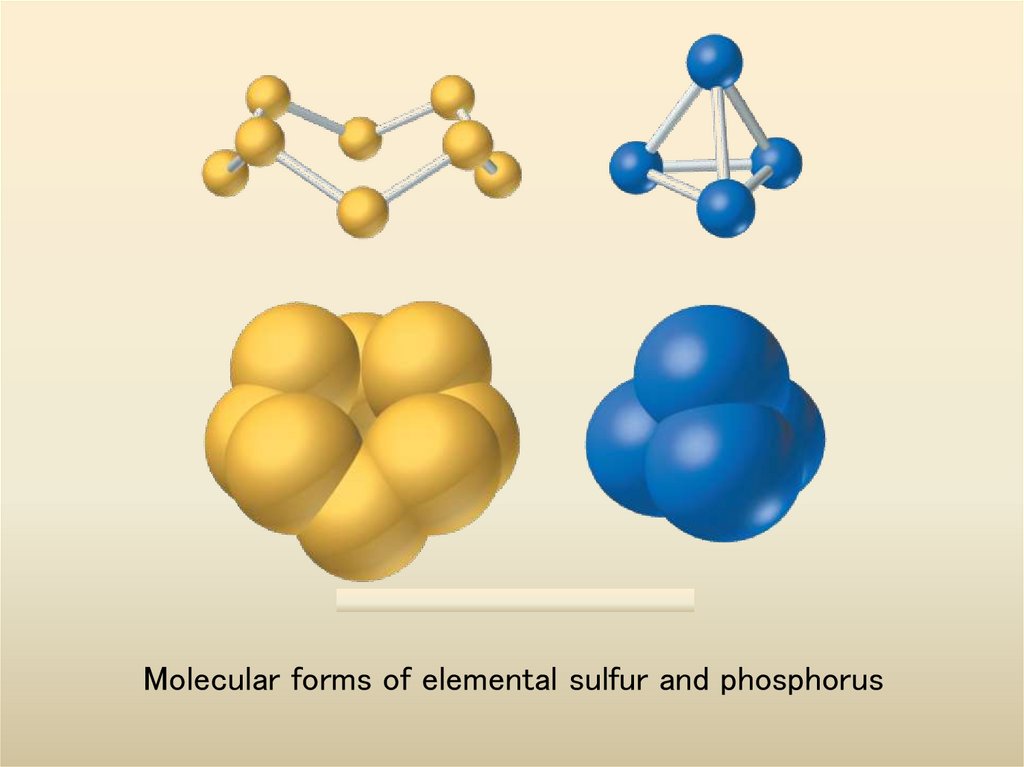
9. Molecular forms of elemental sulfur and phosphorus
10. 3-2 The Mole Concept and Chemical Compounds
Formula massthe mass of a formula unit in atomic mass
units (u)
Molecular mass
a formula mass of a molecular compound
Weighted average mass
add up the weighted average atomic
masses
Exact Mass
add up the isotopic masses (see mass
spectrometry)
KEEP IN MIND
that although molecular
mass and molar mass
sound similar and are
related, they are not
the same. Molecular mass
is the weighted-average
mass of one molecule
expressed in atomic mass
units, u. Molar mass is the
mass of Avogadro’s number
of molecules expressed in
grams per mole, The two
terms have the same
numerical value but different
units. g/mol.
11.
Examples:1. An analytical balance can detect a mass of 0.1 mg. What is the total
number of ions present in this minimally detectable quantity of MgCl2?
2.
Zinc oxide, ZnO is used in sunscreen preparation. What is the total
number of ions present in a 1 g of ZnO?
3. How many grams of would you need to obtain ions?
4. The volatile liquid ethyl mercaptan C2H6S is one of the most odoriferous
substances known. It is used in natural gas he make gas leaks detectable.
How many C2H6S molecules are contained in a 1 microliter sample? (d:
0.84g/ml)
5. Gold has a density of 19.32 g/cm3. A piece of gold leaf is 2.5 cm on each
side and 0.1 mm thick. How many atoms of gold are in this piece of gold
leaf?
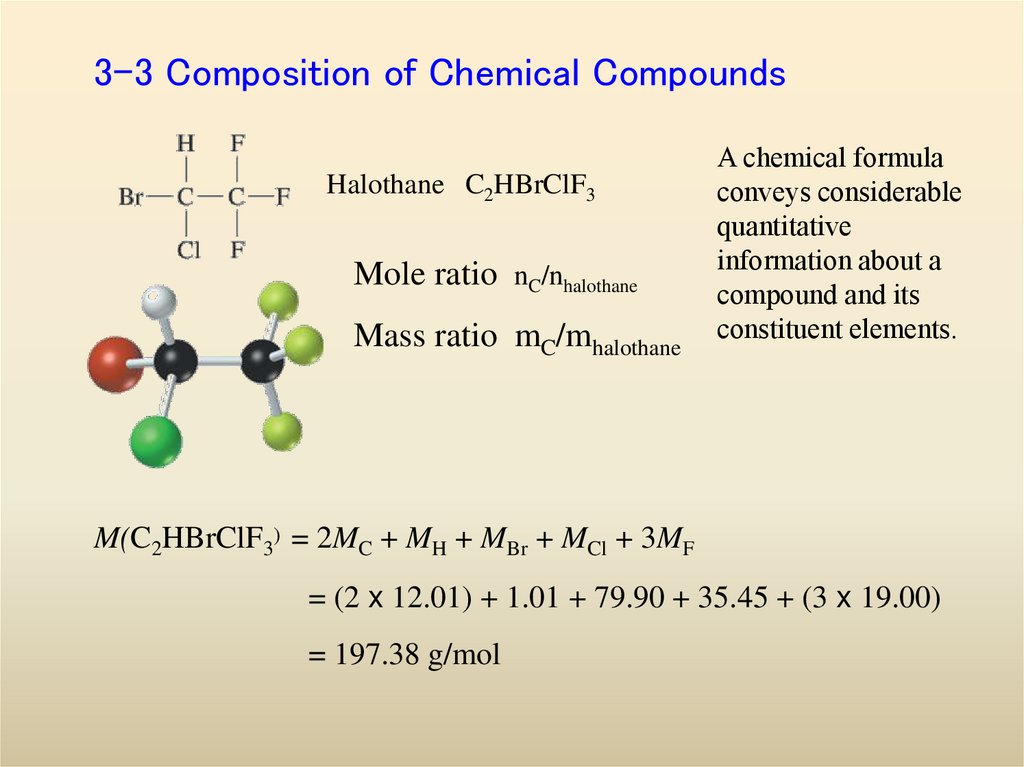
12. 3-3 Composition of Chemical Compounds
Halothane C2HBrClF3Mole ratio nC/nhalothane
Mass ratio mC/mhalothane
A chemical formula
conveys considerable
quantitative
information about a
compound and its
constituent elements.
M(C2HBrClF3) = 2MC + MH + MBr + MCl + 3MF
= (2 x 12.01) + 1.01 + 79.90 + 35.45 + (3 x 19.00)
= 197.38 g/mol
13.
Examples:1. How many moles of F atoms are in a 75 ml sample of halothane
C2HBrClF3? (d: 1.871g/ml) C2HBrClF3=197.4 g/mol
2.
How many grams of C are contained in 75 ml of halothane?
3.
What is the mass percent composition of halothane?
14.
The percentages of the elements in a compound should add up to 100%, andwe can use this fact in one of two ways;
1.
Check the accuracy of the compotations by ensuring that the percentages
do total 100%.
2.
Determine the percenteges of all the elements but one. Obtain that one by
difference.
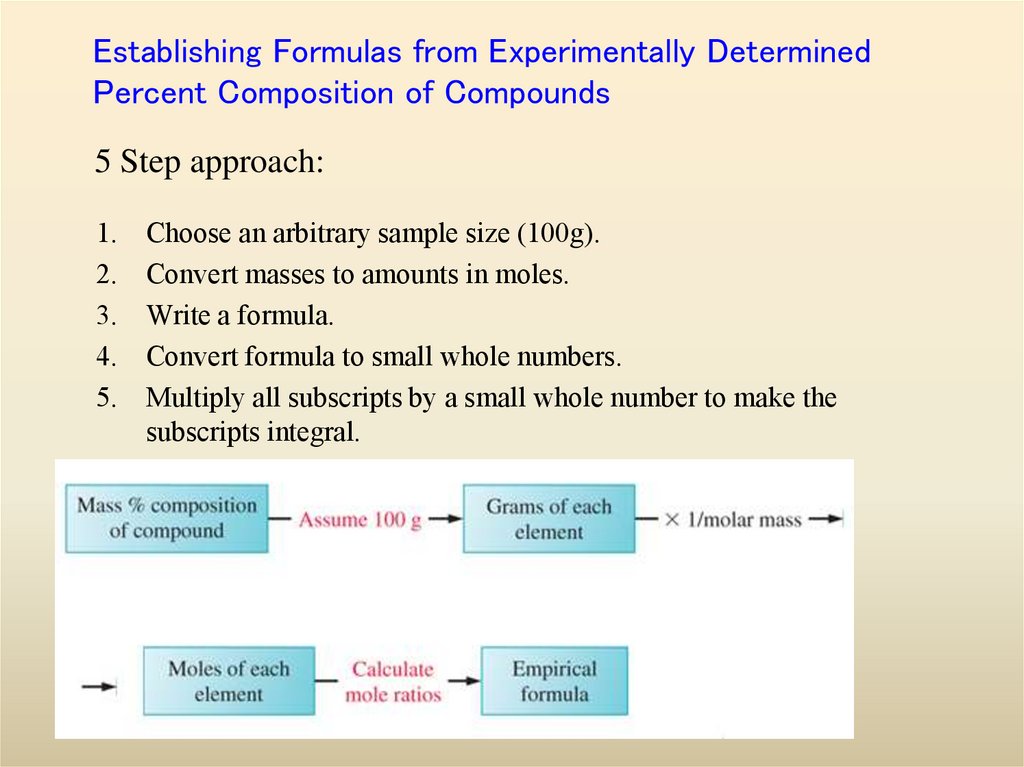
15. Establishing Formulas from Experimentally Determined Percent Composition of Compounds
5 Step approach:1.
2.
3.
4.
5.
Choose an arbitrary sample size (100g).
Convert masses to amounts in moles.
Write a formula.
Convert formula to small whole numbers.
Multiply all subscripts by a small whole number to make the
subscripts integral.
16.
Examples:1. Dibutyl succinate is an insect repellent used against household ants and
roaches. Its composition is 62.58% C, and 27.79% O. Its experimentally
determined molecular mass is 230 u. What are the empirical and
molecular formulas of dibtyl succinate?
2.
Diacetoneglucose has a molecular mass of 260 u and the mass present
composition: 55.37% C, 7.75% H and 36.88% O. What are the empirical
and molecular formulas of this substance?
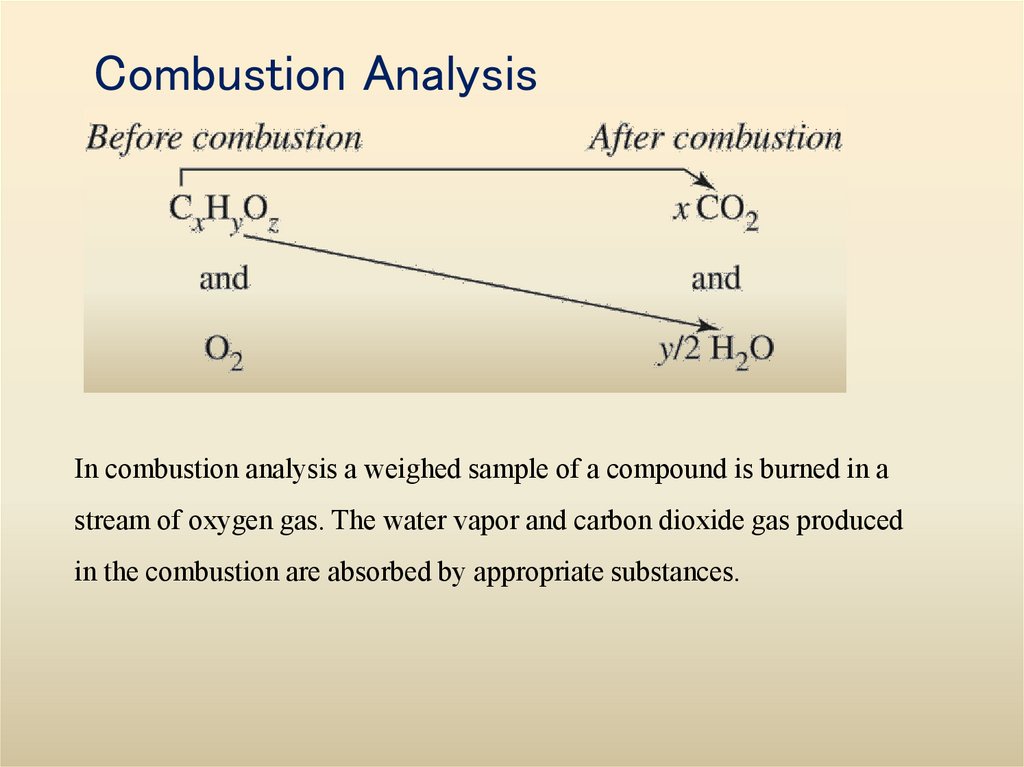
17. Combustion Analysis
In combustion analysis a weighed sample of a compound is burned in astream of oxygen gas. The water vapor and carbon dioxide gas produced
in the combustion are absorbed by appropriate substances.
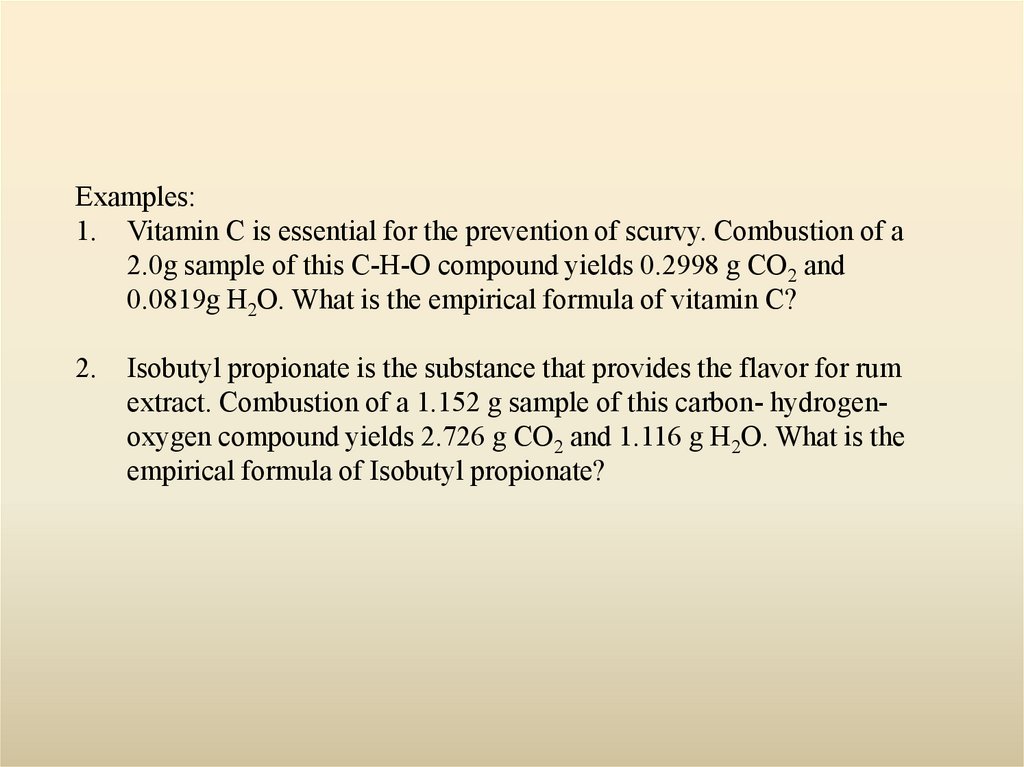
18.
Examples:1. Vitamin C is essential for the prevention of scurvy. Combustion of a
2.0g sample of this C-H-O compound yields 0.2998 g CO2 and
0.0819g H2O. What is the empirical formula of vitamin C?
2.
Isobutyl propionate is the substance that provides the flavor for rum
extract. Combustion of a 1.152 g sample of this carbon- hydrogenoxygen compound yields 2.726 g CO2 and 1.116 g H2O. What is the
empirical formula of Isobutyl propionate?
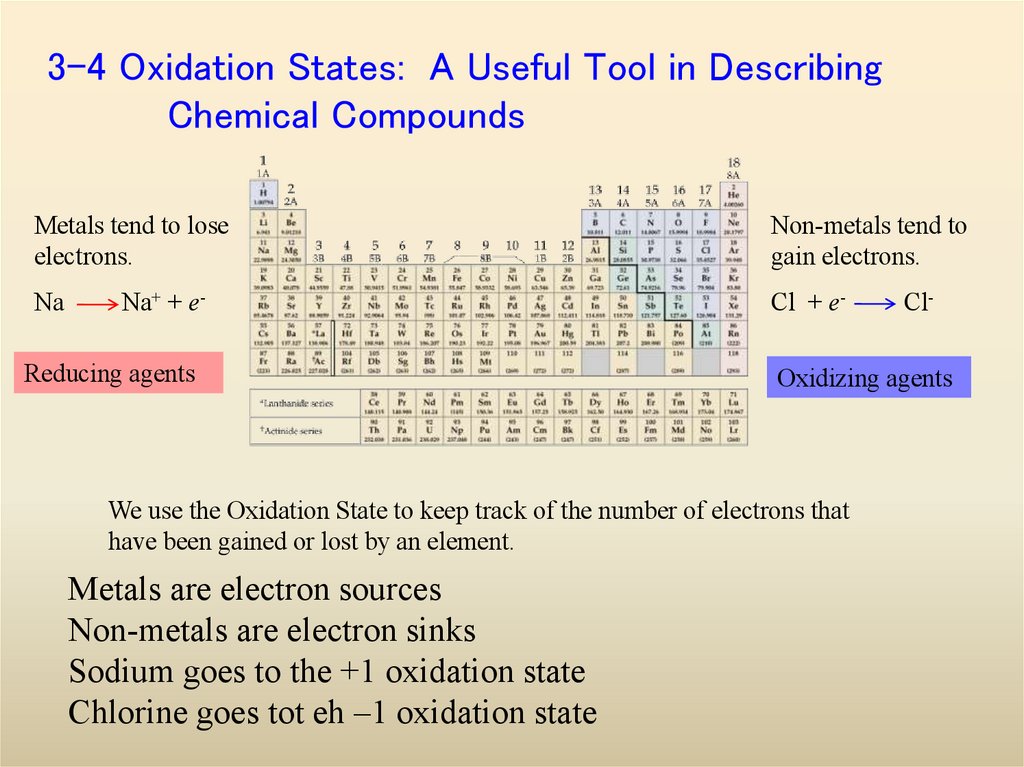
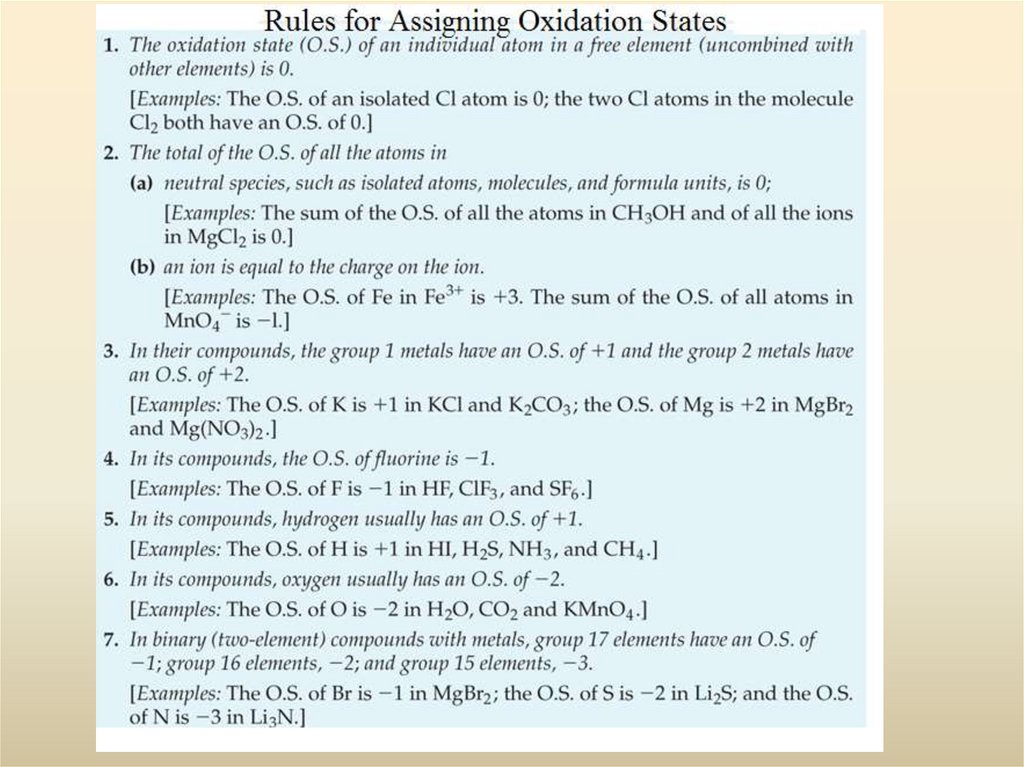
19. 3-4 Oxidation States: A Useful Tool in Describing Chemical Compounds
Metals tend to loseelectrons.
Na
Na+ + e-
Reducing agents
Non-metals tend to
gain electrons.
Cl + e-
Oxidizing agents
We use the Oxidation State to keep track of the number of electrons that
have been gained or lost by an element.
Metals are electron sources
Non-metals are electron sinks
Sodium goes to the +1 oxidation state
Chlorine goes tot eh –1 oxidation state
Cl-
20.
21.
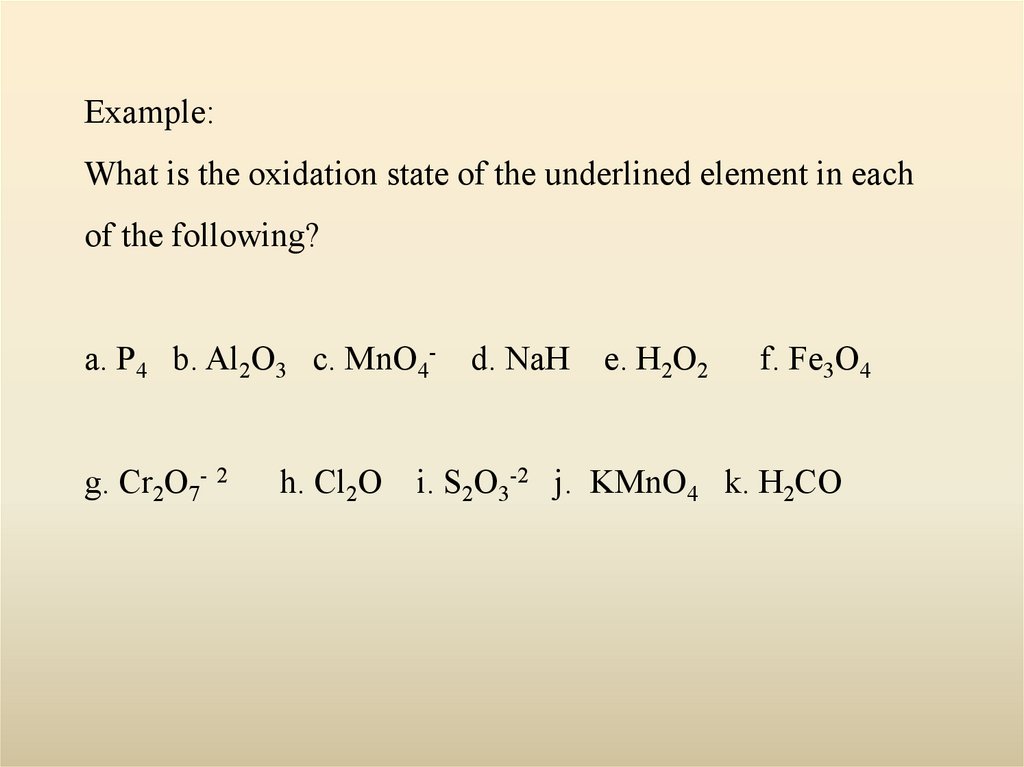
Example:What is the oxidation state of the underlined element in each
of the following?
a. P4 b. Al2O3 c. MnO4g. Cr2O7- 2
h. Cl2O
d. NaH
e. H2O2
f. Fe3O4
i. S2O3-2 j. KMnO4 k. H2CO
22. 3-5 Naming Compounds: Organic and Inorganic Compounds
Lead (IV) oxideTwo oxides of lead
Lead (II) oxide
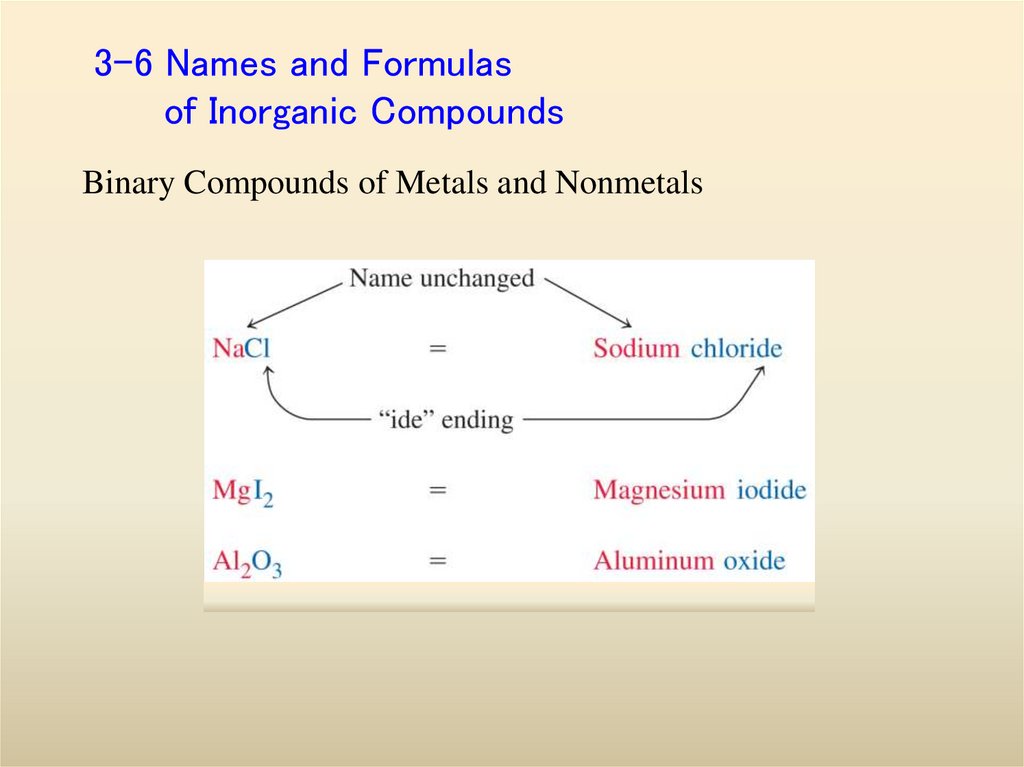
23. 3-6 Names and Formulas of Inorganic Compounds
Binary Compounds of Metals and Nonmetals24.
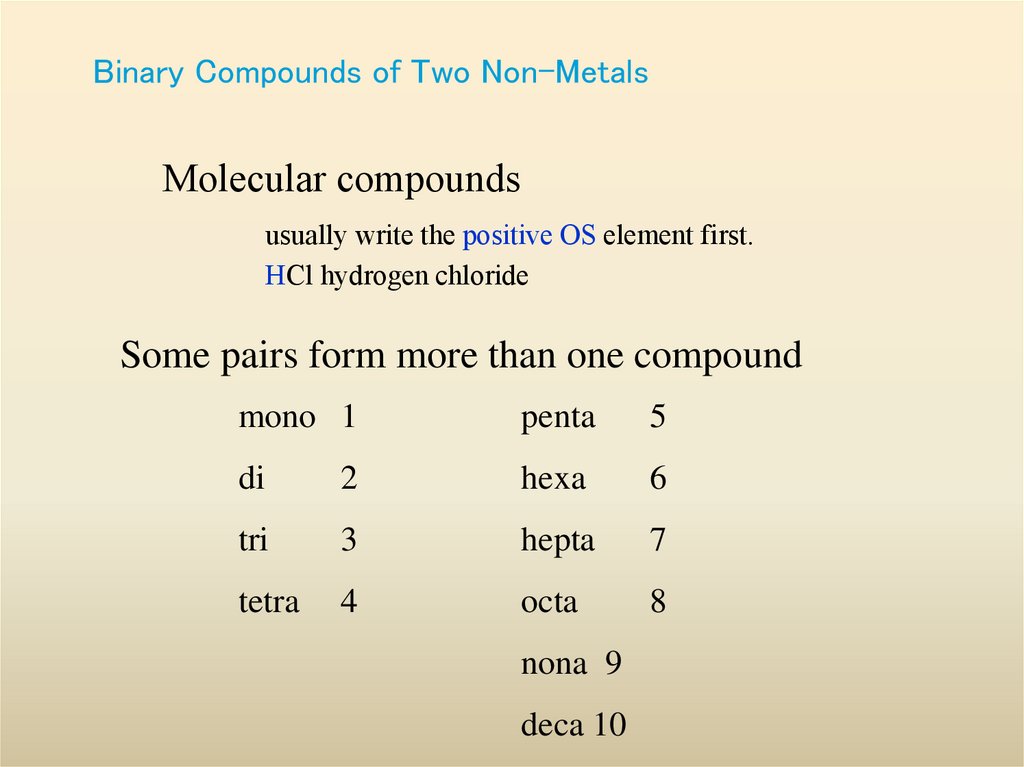
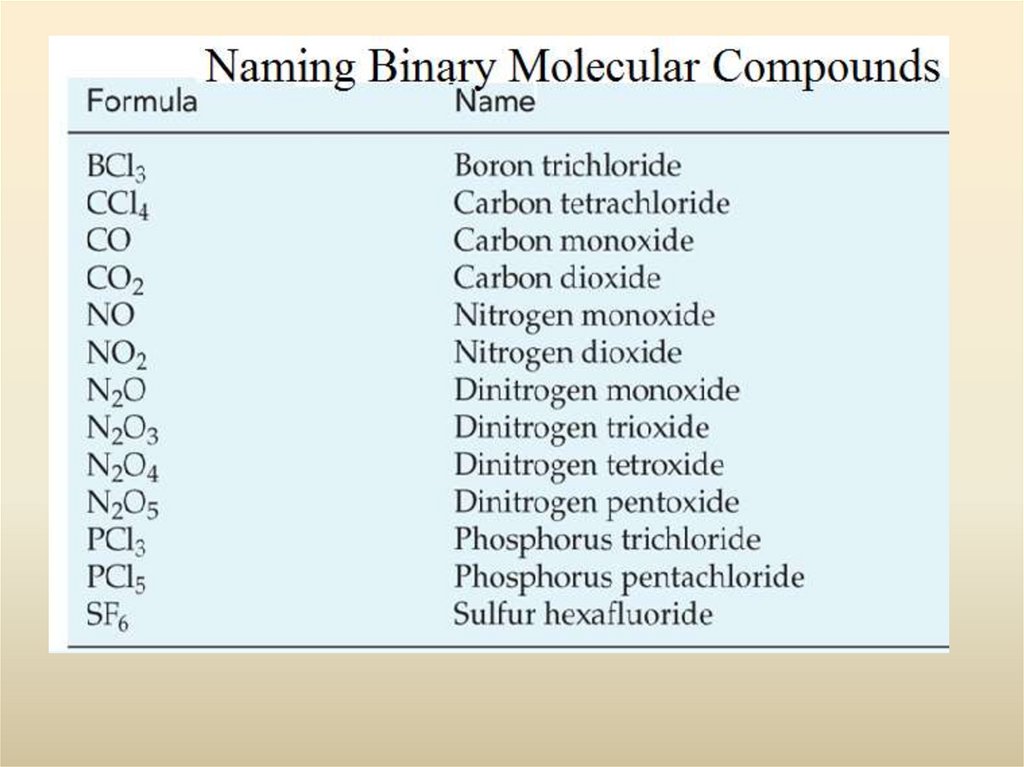
25. Binary Compounds of Two Non-Metals
Molecular compoundsusually write the positive OS element first.
HCl hydrogen chloride
Some pairs form more than one compound
mono 1
penta
5
di
2
hexa
6
tri
3
hepta
7
tetra
4
octa
8
nona 9
deca 10
26.
27. Binary Acids
Acids produce H+ when dissolved in water.They are compounds that ionize in water.
The symbol (aq) signifies aqueous solution.
H2S(aq) = hydrosulfuric acid
HI(aq) = hydroiodic acid
HCl(aq) = hydrochloric acid
HBr(aq) = hydrobromic acid
HF(aq) = hydrofluoric acid
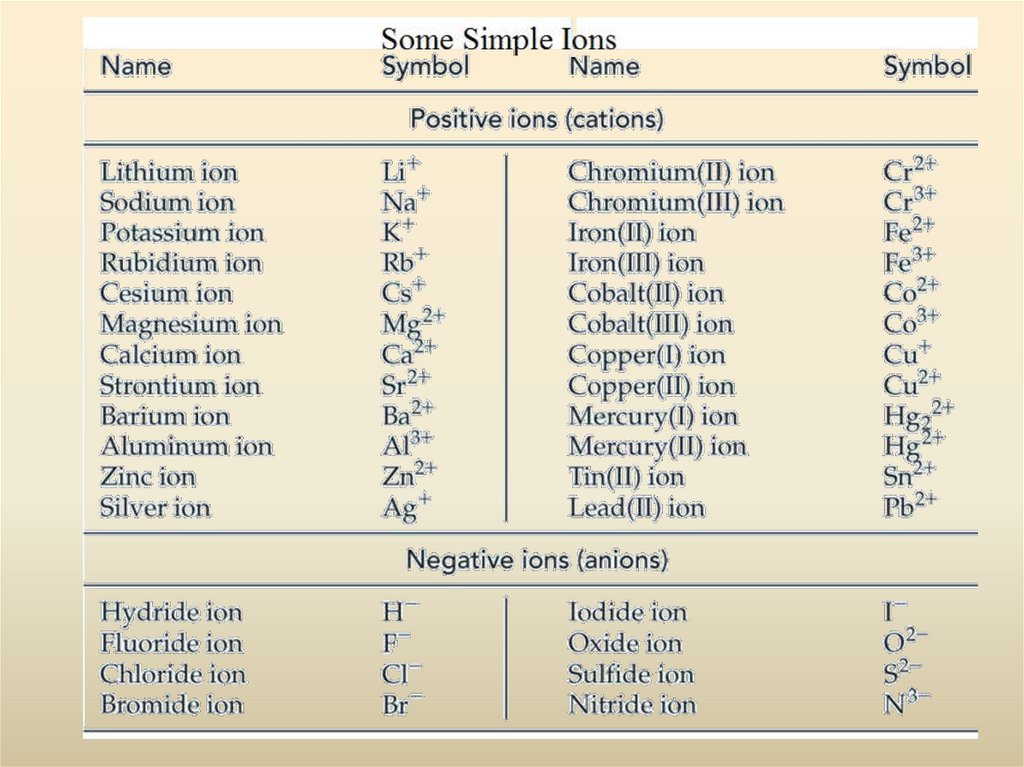
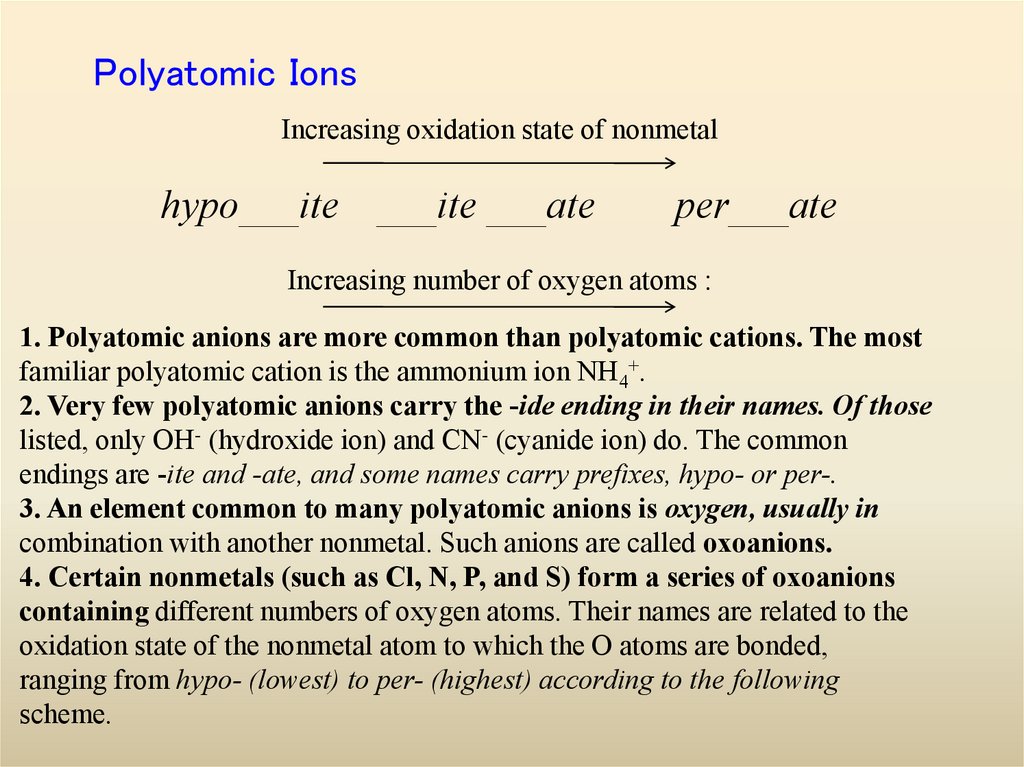
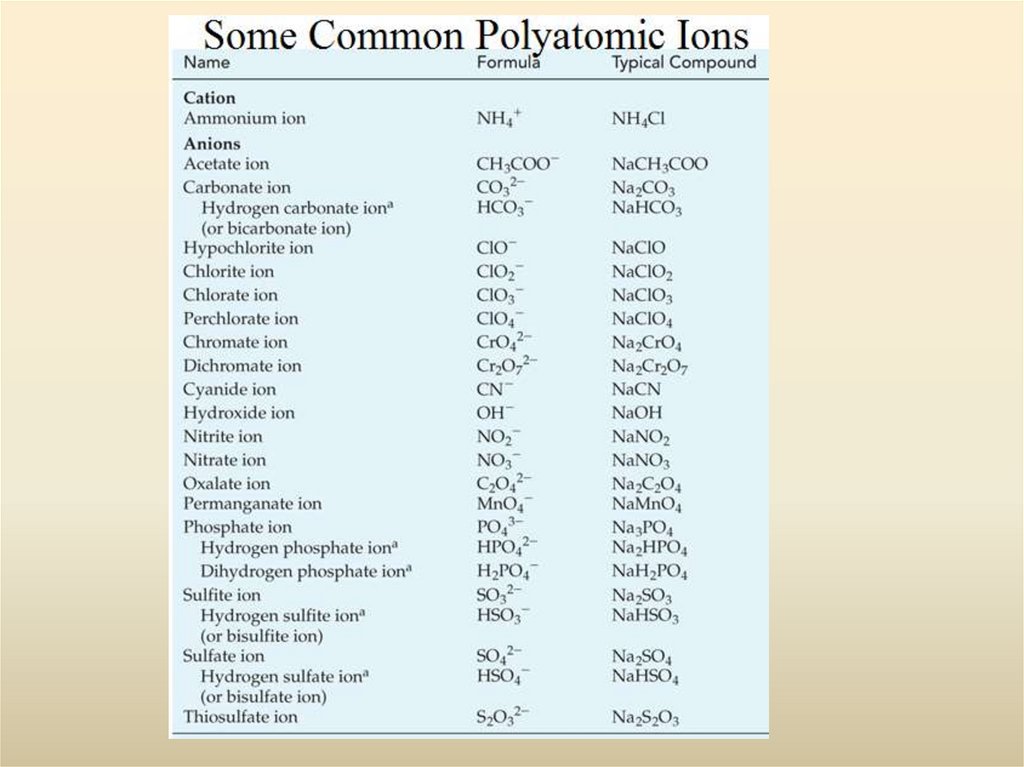
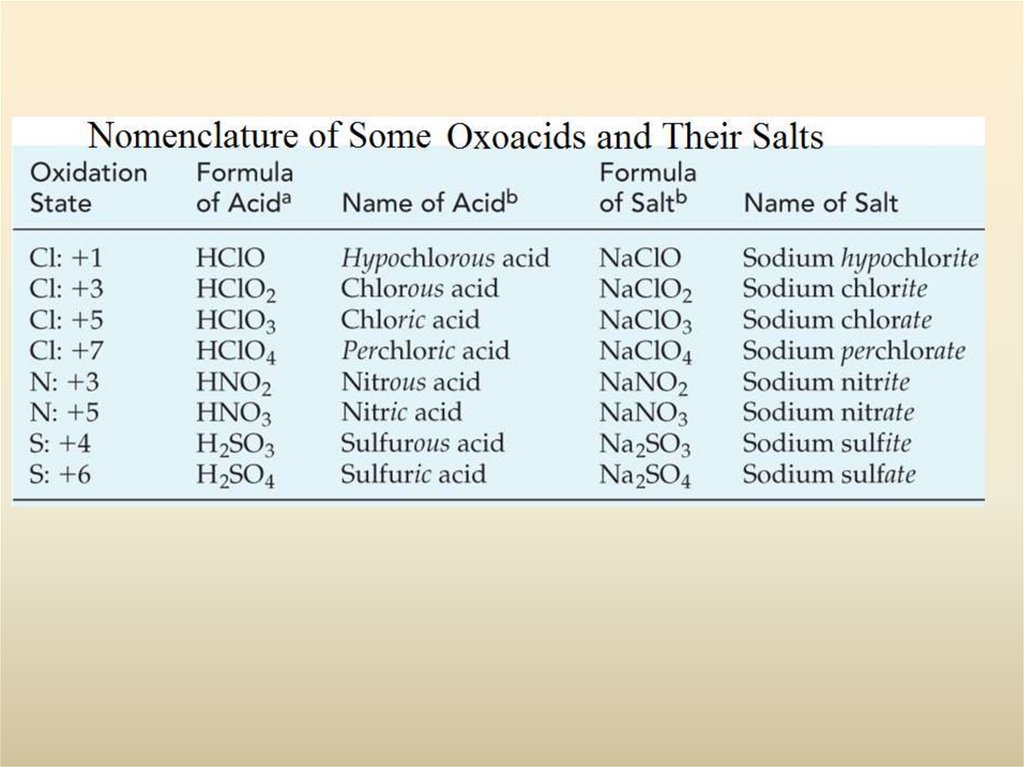
28. Polyatomic Ions
Increasing oxidation state of nonmetalhypo___ite ___ite ___ate
per___ate
Increasing number of oxygen atoms :
1. Polyatomic anions are more common than polyatomic cations. The most
familiar polyatomic cation is the ammonium ion NH4+.
2. Very few polyatomic anions carry the -ide ending in their names. Of those
listed, only OH- (hydroxide ion) and CN- (cyanide ion) do. The common
endings are -ite and -ate, and some names carry prefixes, hypo- or per-.
3. An element common to many polyatomic anions is oxygen, usually in
combination with another nonmetal. Such anions are called oxoanions.
4. Certain nonmetals (such as Cl, N, P, and S) form a series of oxoanions
containing different numbers of oxygen atoms. Their names are related to the
oxidation state of the nonmetal atom to which the O atoms are bonded,
ranging from hypo- (lowest) to per- (highest) according to the following
scheme.
29.
30.
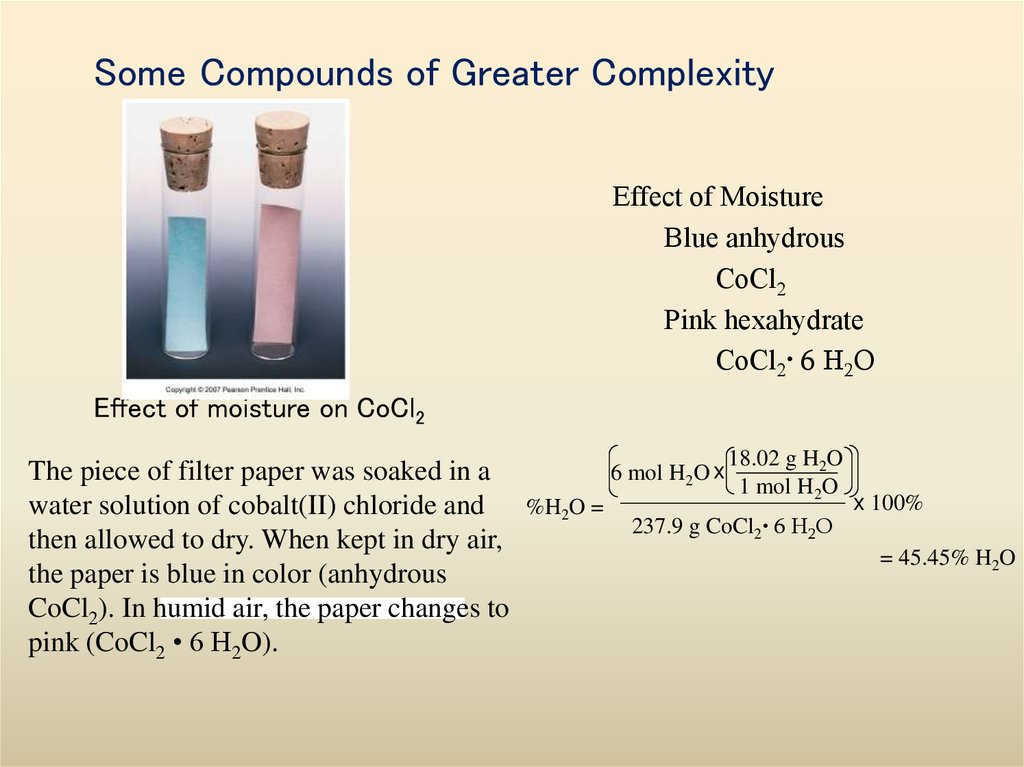
31. Some Compounds of Greater Complexity
Effect of MoistureBlue anhydrous
CoCl2
Pink hexahydrate
CoCl2• 6 H2O
Effect of moisture on CoCl2
18.02 g H O
2
6 mol H2O x
The piece of filter paper was soaked in a
1 mol H2O
x 100%
water solution of cobalt(II) chloride and %H2O =
237.9 g CoCl2• 6 H2O
then allowed to dry. When kept in dry air,
= 45.45% H2O
the paper is blue in color (anhydrous
CoCl2). In humid air, the paper changes to
pink (CoCl2 • 6 H2O).
32. 3-7 Names and Formulas of Organic Compounds
Organic compounds abound in natureFats, carbohydrates and proteins are foods.
Propane, gasoline, kerosene, oil are fuels.
Drugs and plastics are produced by chemical industries.
Carbon atoms form chains and rings and act as
the framework of molecules.
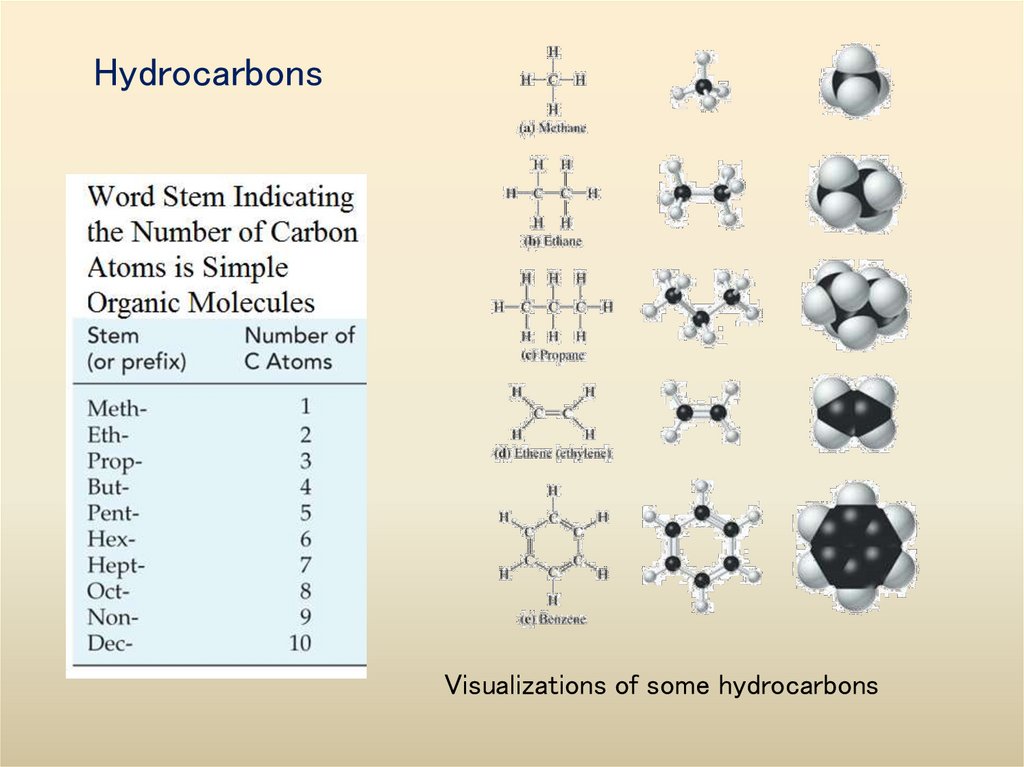
33. Hydrocarbons
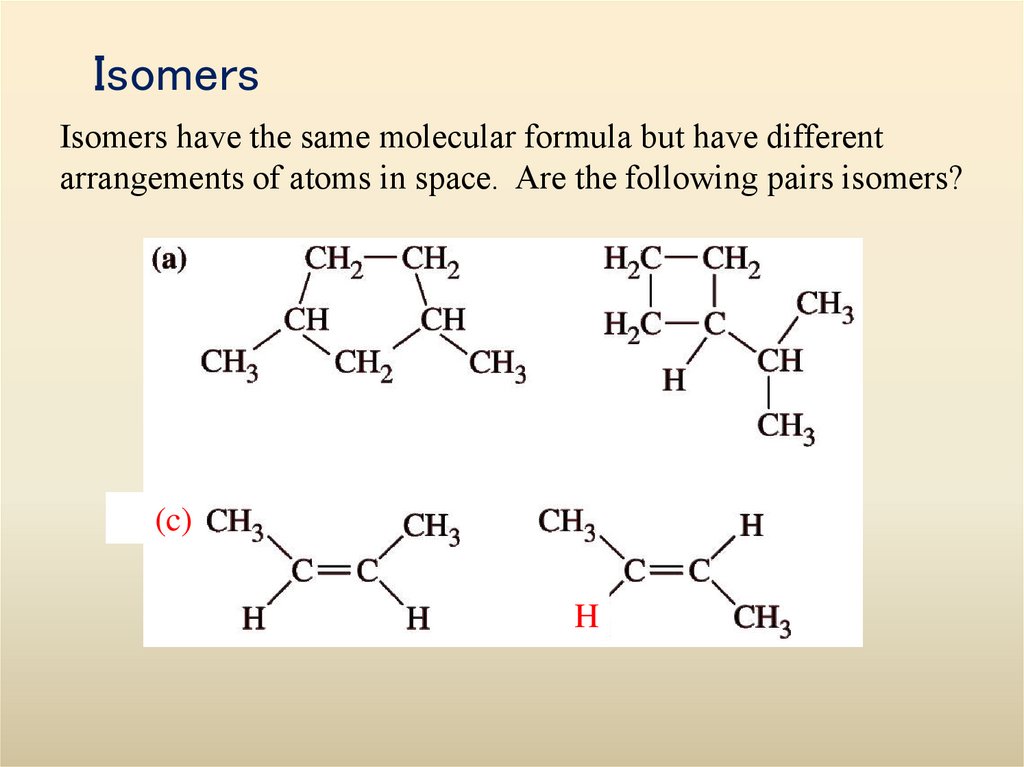
Visualizations of some hydrocarbons34. Isomers
Isomers have the same molecular formula but have differentarrangements of atoms in space. Are the following pairs isomers?
(c)
H
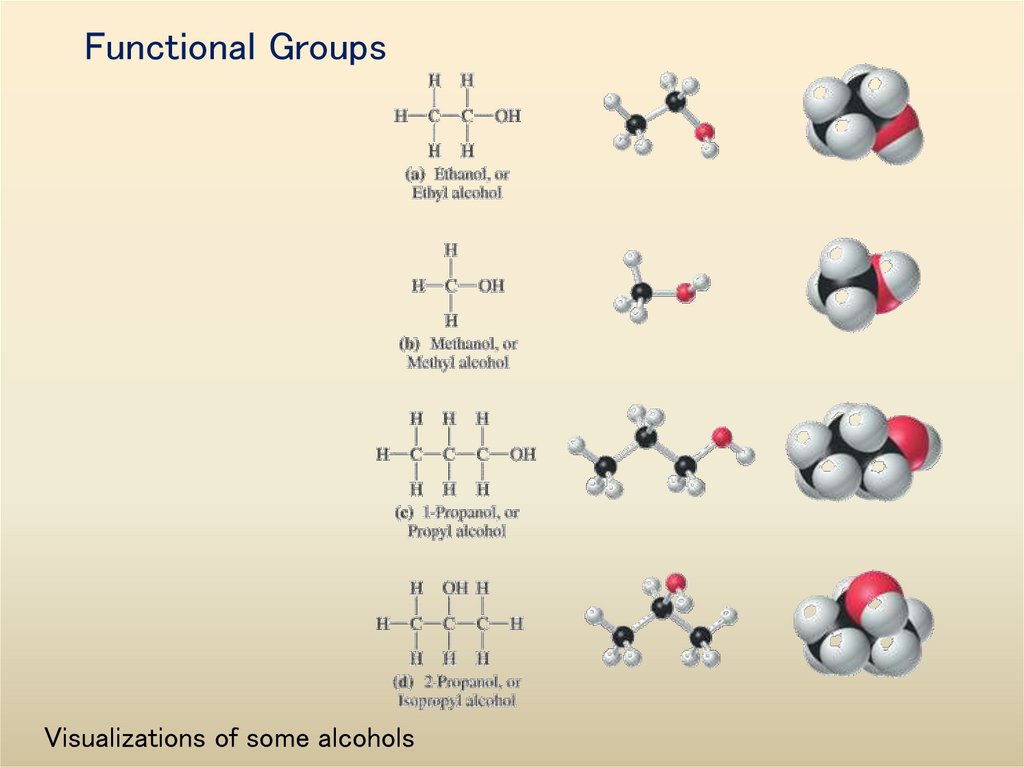
35. Functional Groups
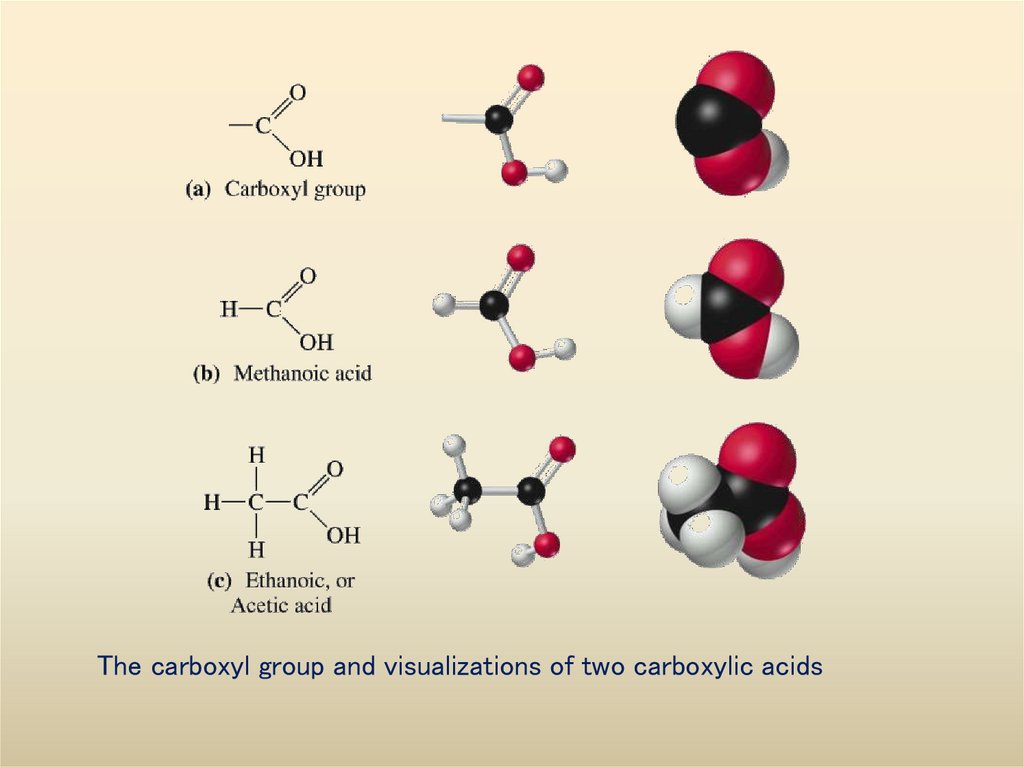
Visualizations of some alcohols36. The carboxyl group and visualizations of two carboxylic acids
37. End of Chapter Questions
Individuals have individual learning styles.You may have more than one style for different
types of learning.
Seeing
Listening
Reading
Writing
Take notes and actively listen.
Participate in your learning process!










 chemistry
chemistry








