Similar presentations:
Revission atomic structure revision (1)
1.
11.1A Atomic structureFundamental particles (p,n,e)
Mass number, atomic (proton) number
isotopes
Relative atomic (molecular, formula) mass
The mass spectrometer
Electronic arrangement
Atomic orbitals and the Aufbau principle
Ionisation energy and the factors affecting it
2.
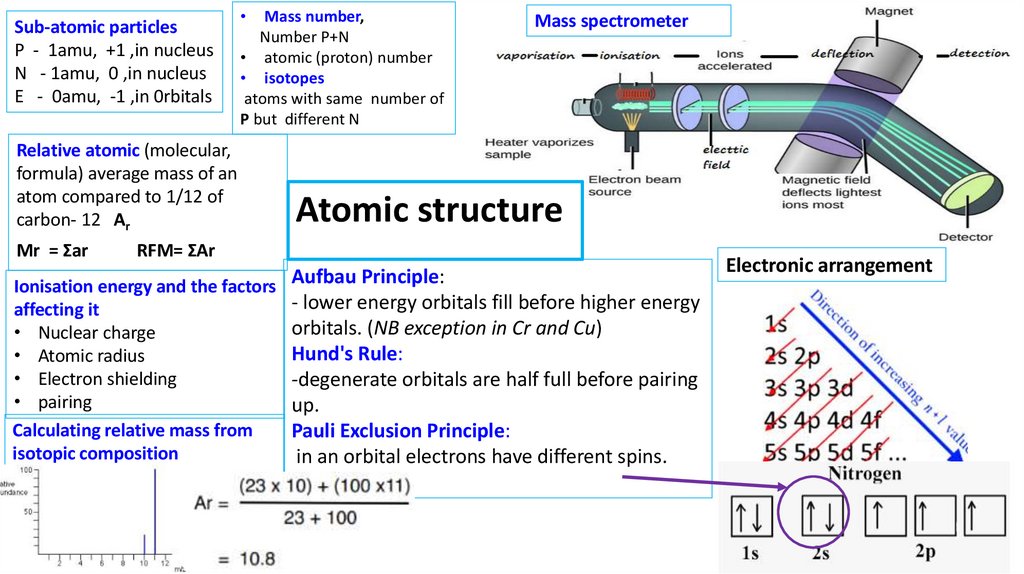
Sub-atomic particlesP - 1amu, +1 ,in nucleus
N - 1amu, 0 ,in nucleus
E - 0amu, -1 ,in 0rbitals
Relative atomic (molecular,
formula) average mass of an
atom compared to 1/12 of
carbon- 12 Ar
Mr = Σar
Mass number,
Number P+N
• atomic (proton) number
• isotopes
atoms with same number of
P but different N
Mass spectrometer
Atomic structure
RFM= ΣAr
Ionisation energy and the factors Aufbau Principle:
- lower energy orbitals fill before higher energy
affecting it
orbitals. (NB exception in Cr and Cu)
• Nuclear charge
Hund's Rule:
• Atomic radius
• Electron shielding
-degenerate orbitals are half full before pairing
• pairing
up.
Calculating relative mass from
Pauli Exclusion Principle:
isotopic composition
in an orbital electrons have different spins.
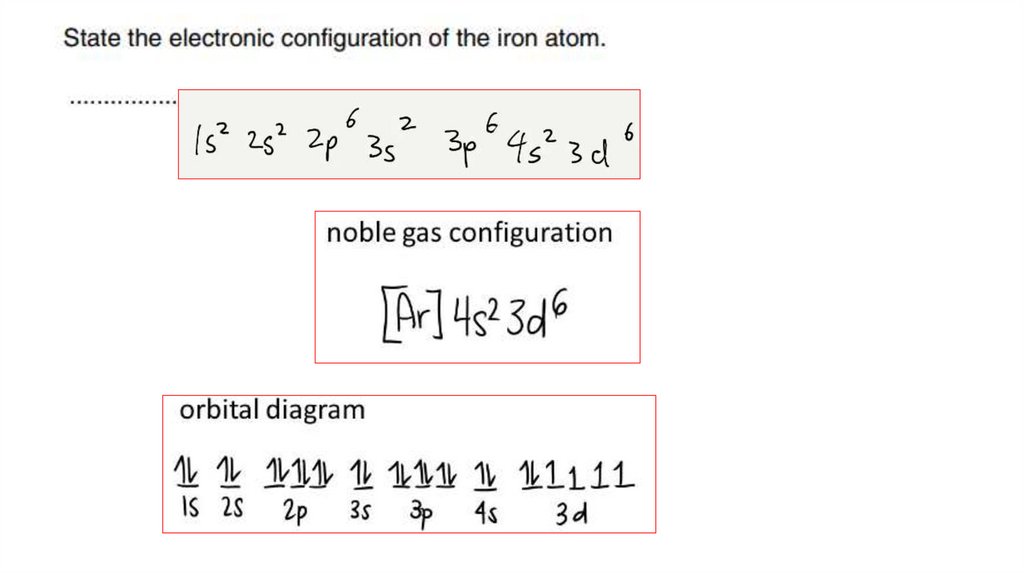
Electronic arrangement
3. Practice questions
*State the number of electrons that each of these canhold
a)A p subshell
b)A p orbital
c)4th shell
*
4. Practice questions
How many possible orbitals are there with n=3?a)1
b)3
c)4
d)5
e)9
*
5. Practice questions
How many possible sub-shells are there with n=3?a)1
b)2
c)3
d)4
e)none
*
6. Practice questions
What orbital is this?a)s
b)p
c)d
d)f
e)none
*
7. Practice questions
Can this orbital be found in n=2 shell?a)yes
b)no
c)maybe
d)No idea
e)I don’t care
*
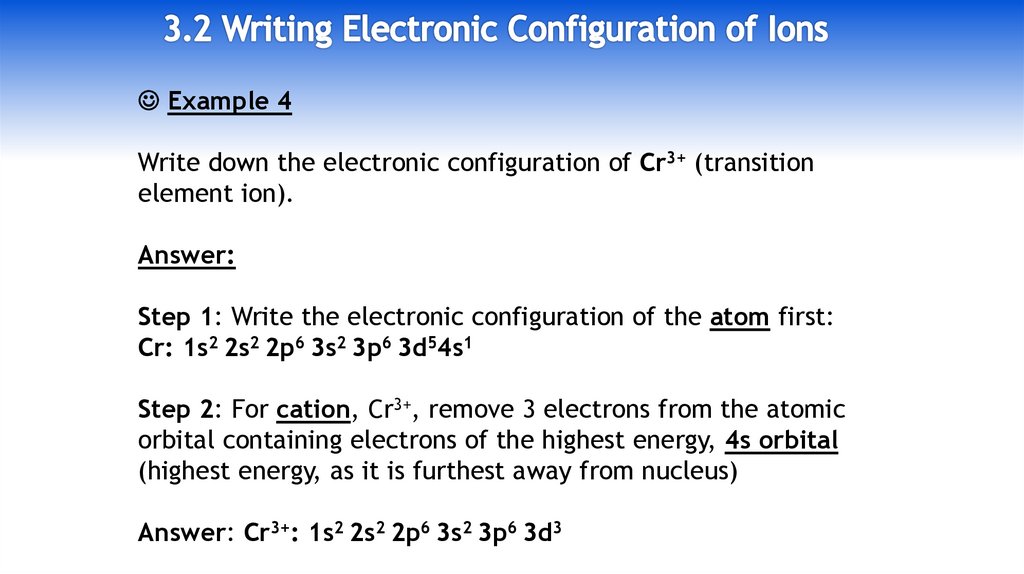
8.
Example 4Write down the electronic configuration of Cr3+ (transition
element ion).
Answer:
Step 1: Write the electronic configuration of the atom first:
Cr: 1s2 2s2 2p6 3s2 3p6 3d54s1
Step 2: For cation, Cr3+, remove 3 electrons from the atomic
orbital containing electrons of the highest energy, 4s orbital
(highest energy, as it is furthest away from nucleus)
9.
Example 4Write down the electronic configuration of Cr3+ (transition
element ion).
Answer:
Step 1: Write the electronic configuration of the atom first:
Cr: 1s2 2s2 2p6 3s2 3p6 3d54s1
Step 2: For cation, Cr3+, remove 3 electrons from the atomic
orbital containing electrons of the highest energy, 4s orbital
(highest energy, as it is furthest away from nucleus)
Answer: Cr3+: 1s2 2s2 2p6 3s2 3p6 3d3
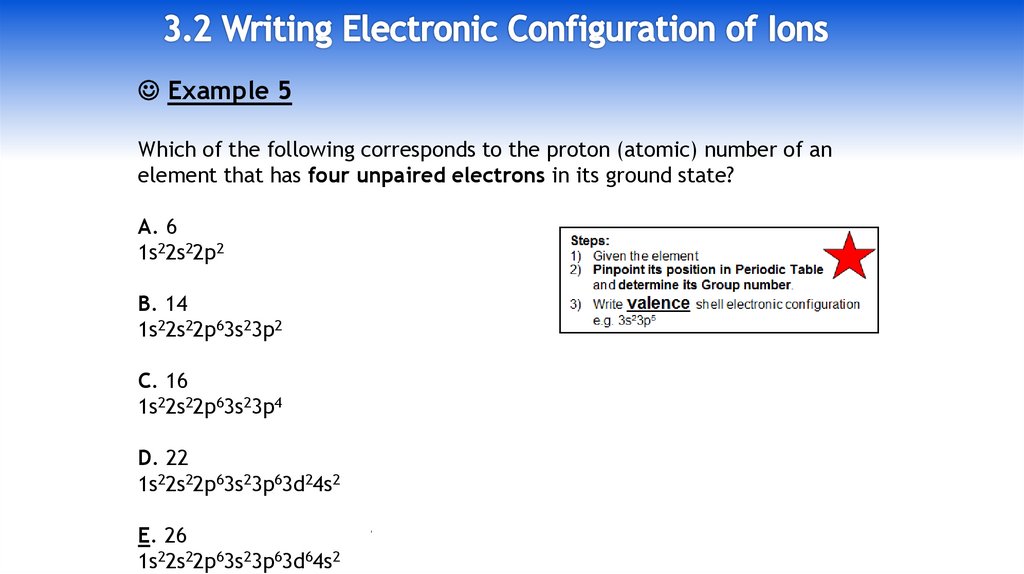
10.
Example 5Which of the following corresponds to the proton (atomic) number of an
element that has four unpaired electrons in its ground state?
A. 6
1s22s22p2
Group IV: 2s22p2
B. 14
1s22s22p63s23p2
Group IV: 3s23p2
C. 16
1s22s22p63s23p4
Group VI: 3s23p4
D. 22
1s22s22p63s23p63d24s2
1st row d-block element: [Ar] 3d24s2
E. 26
1s22s22p63s23p63d64s2
1st row d-block element: [Ar] 3d64s2
11.
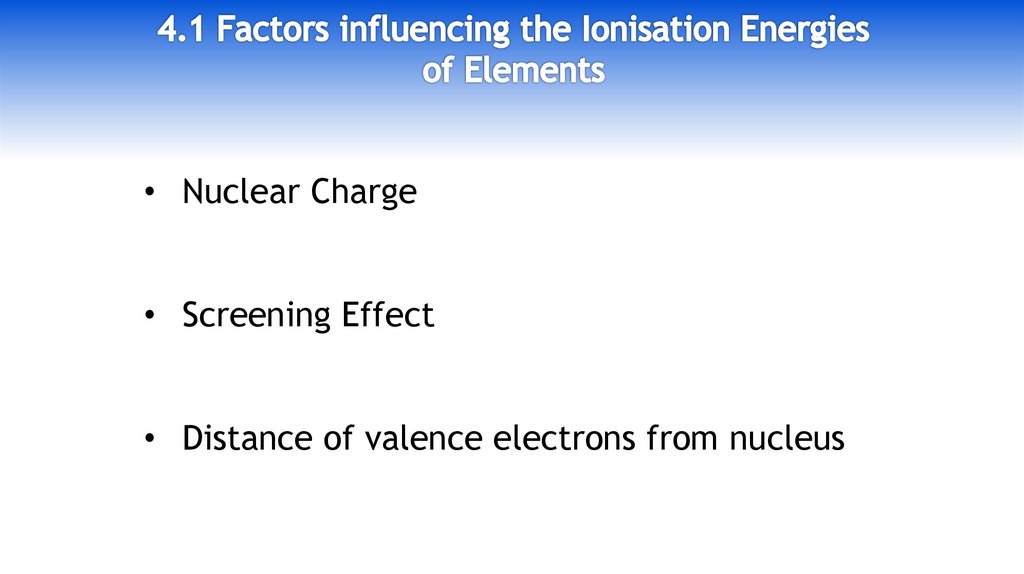
• Nuclear Charge• Screening Effect
• Distance of valence electrons from nucleus
12.
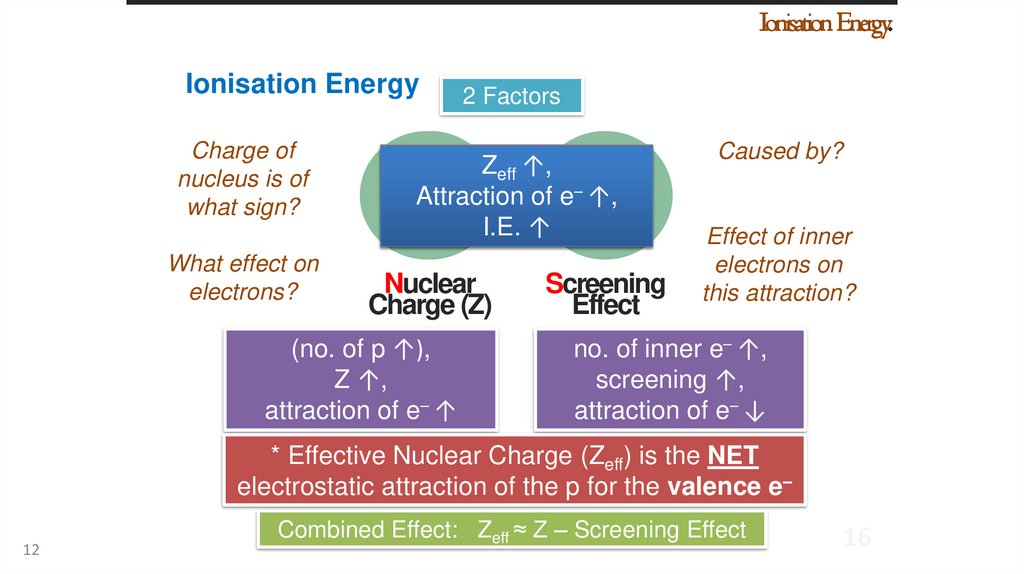
Ionisation Energy.Ionisation Energy
Charge of
nucleus is of
what sign?
What effect on
electrons?
2 Factors
Zeff ↑,
Attraction of e– ↑,
Atomic/Ionic
I.E. ↑radius ↓
Nuclear
Charge (Z)
(no. of p ↑),
Z ↑,
attraction of e– ↑
Screening
Effect
Caused by?
Effect of inner
electrons on
this attraction?
no. of inner e– ↑,
screening ↑,
attraction of e– ↓
* Effective Nuclear Charge (Zeff) is the NET
electrostatic attraction of the p for the valence e–
12
Combined Effect: Zeff ≈ Z – Screening Effect
16
13.
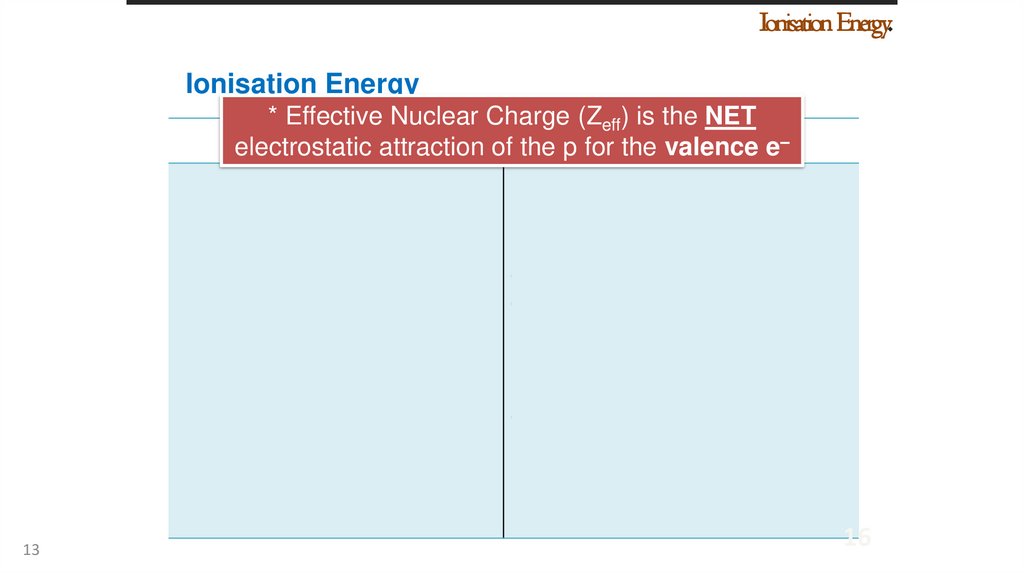
Ionisation Energy.Ionisation Energy
* Effective Nuclear Charge (Zeff) is the NET
Nuclear
charge
(Z)
Screening
effecte–
electrostatic
attraction
of the p for
the valence
Nuclear charge ↑, I.E. ↑
Screening effect ↑, I.E. ↓
As nuclear charge increases, As screening effect increases,
attraction
for
valence attraction
for
valence
electrons increases (more electrons decreases (less
strongly attracted to the strongly
attracted
to
the
nucleus)
nucleus)
More energy is required to
remove an electron
Less energy is required to
remove an electron
Ionisation energy increases. Ionisation energy decreases.
13
16
14.
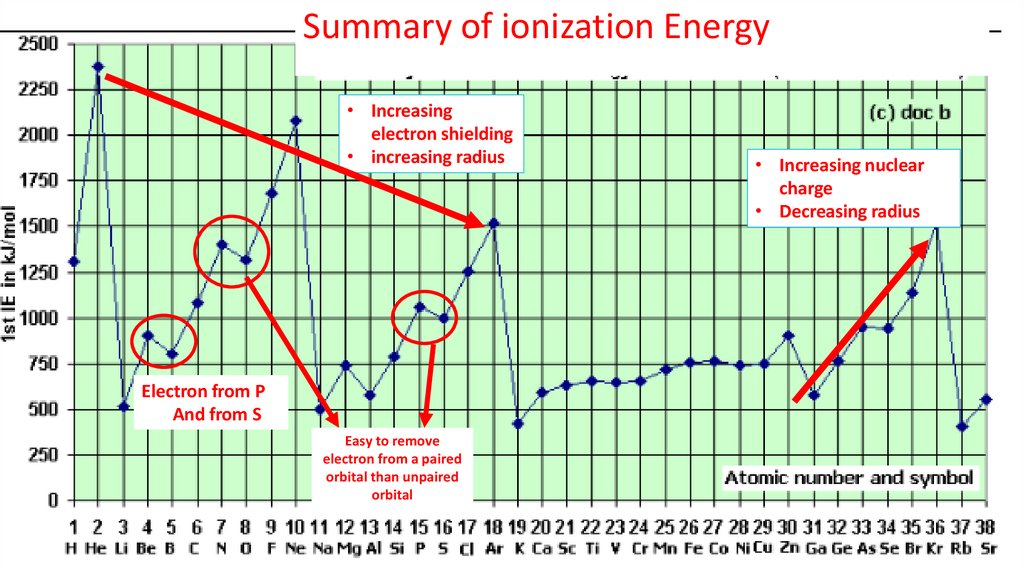
Summary of ionization Energy• Increasing
electron shielding
• increasing radius
Electron from P
And from S
Easy to remove
electron from a paired
orbital than unpaired
orbital
• Increasing nuclear
charge
• Decreasing radius
15.
Explanation• Nuclear charge increases as the number of protons increases
• Shielding effect remains relatively constant as the number
of inner core electrons remains the same
• Effective nuclear charge increases
• Valence electrons are more strongly attracted to the nucleus
16.
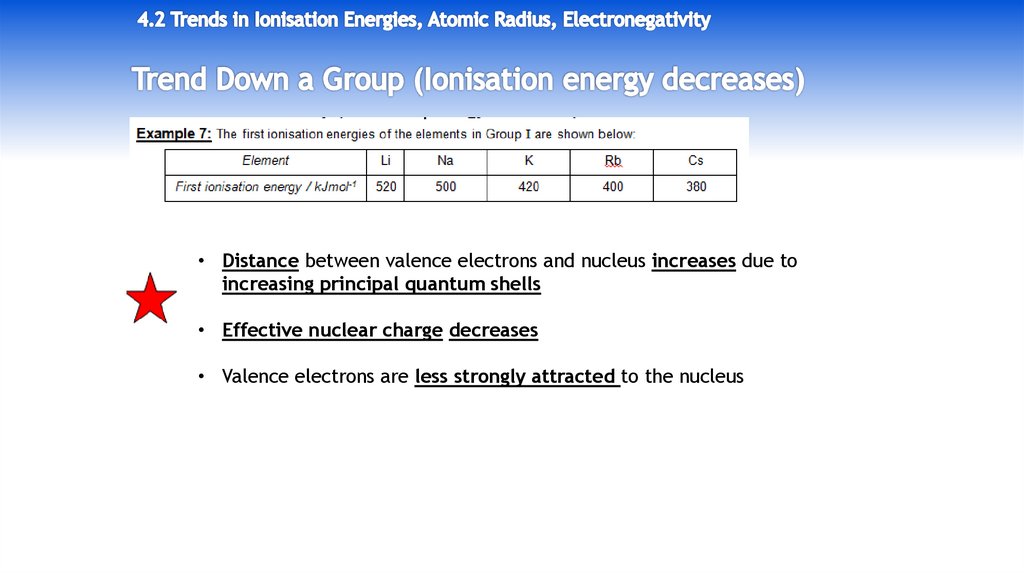
• Distance between valence electrons and nucleus increases due toincreasing principal quantum shells
• Effective nuclear charge decreases
• Valence electrons are less strongly attracted to the nucleus
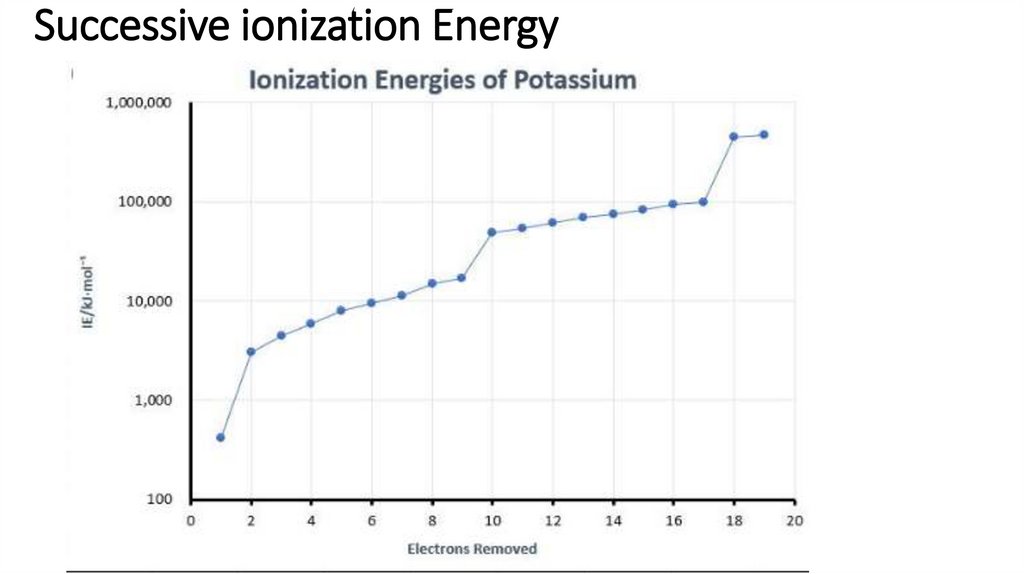
17. Successive ionization Energy
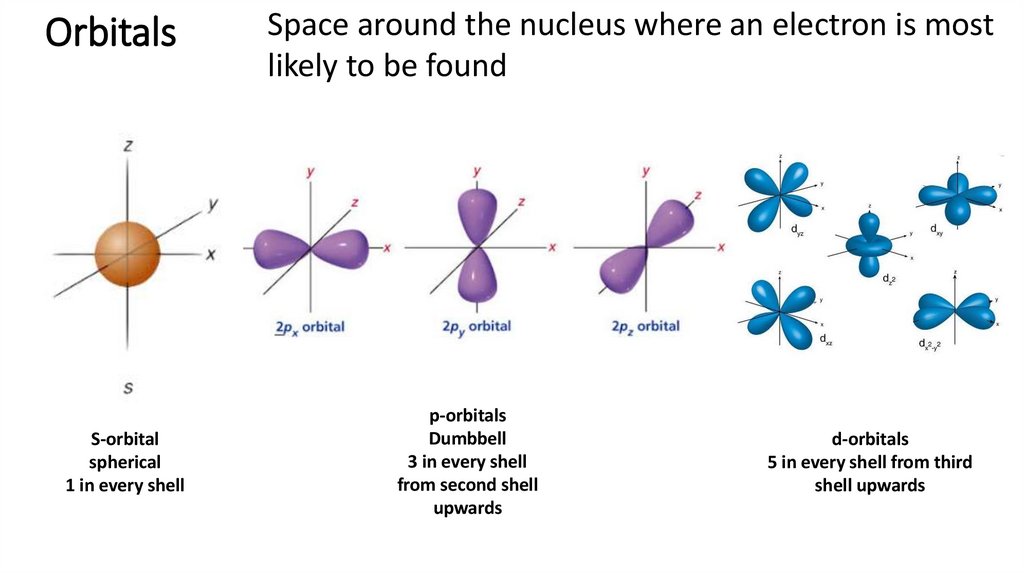
18. Orbitals
S-orbitalspherical
1 in every shell
Space around the nucleus where an electron is most
likely to be found
p-orbitals
Dumbbell
3 in every shell
from second shell
upwards
d-orbitals
5 in every shell from third
shell upwards
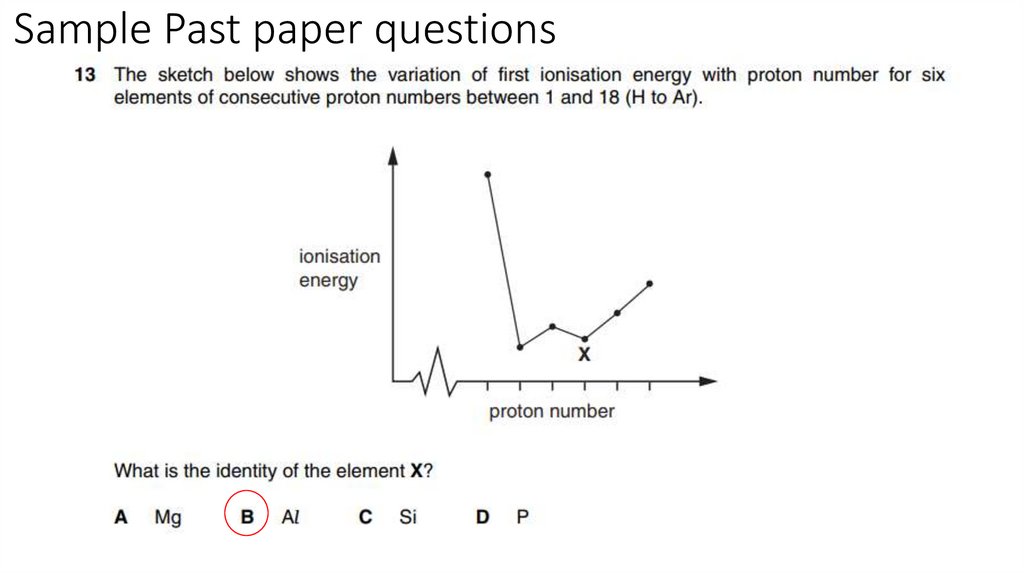
19. Sample Past paper questions
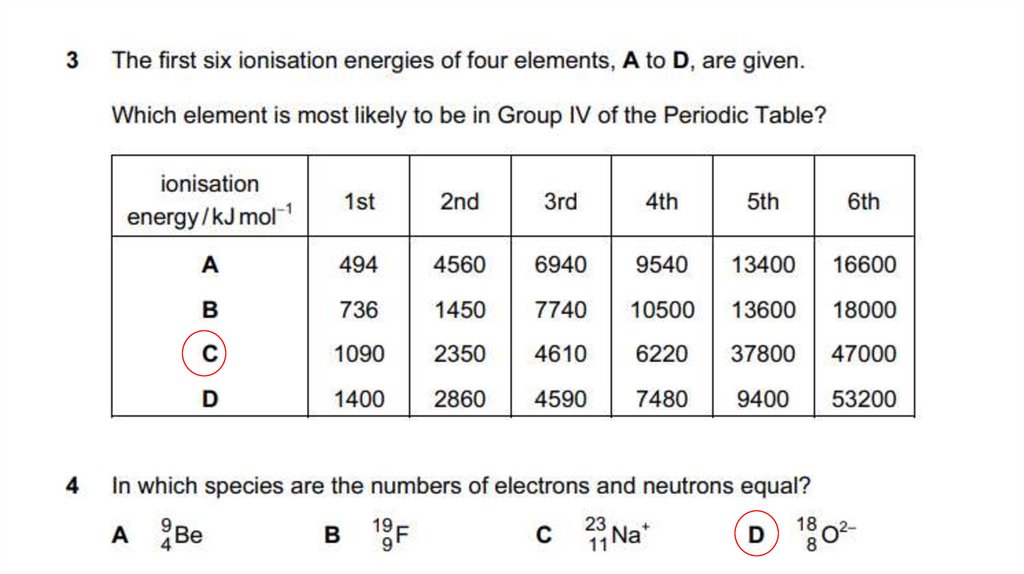
20. Sample Past paper questions
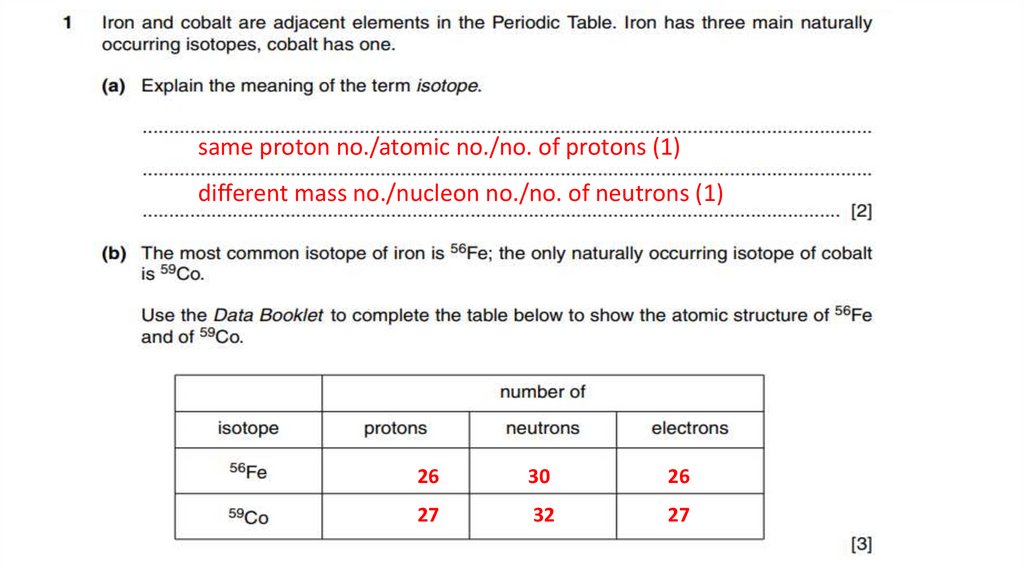
21. Sample Past paper questions
22.
23.
same proton no./atomic no./no. of protons (1)different mass no./nucleon no./no. of neutrons (1)
26
30
26
27
32
27
24.
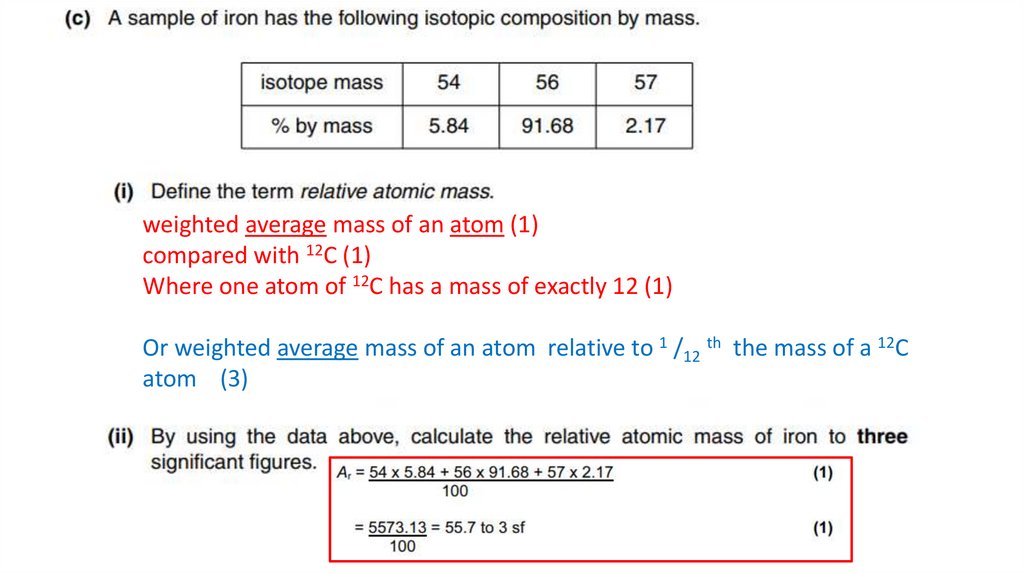
weighted average mass of an atom (1)compared with 12C (1)
Where one atom of 12C has a mass of exactly 12 (1)
Or weighted average mass of an atom relative to 1 /12 th the mass of a 12C
atom (3)
25.
26.
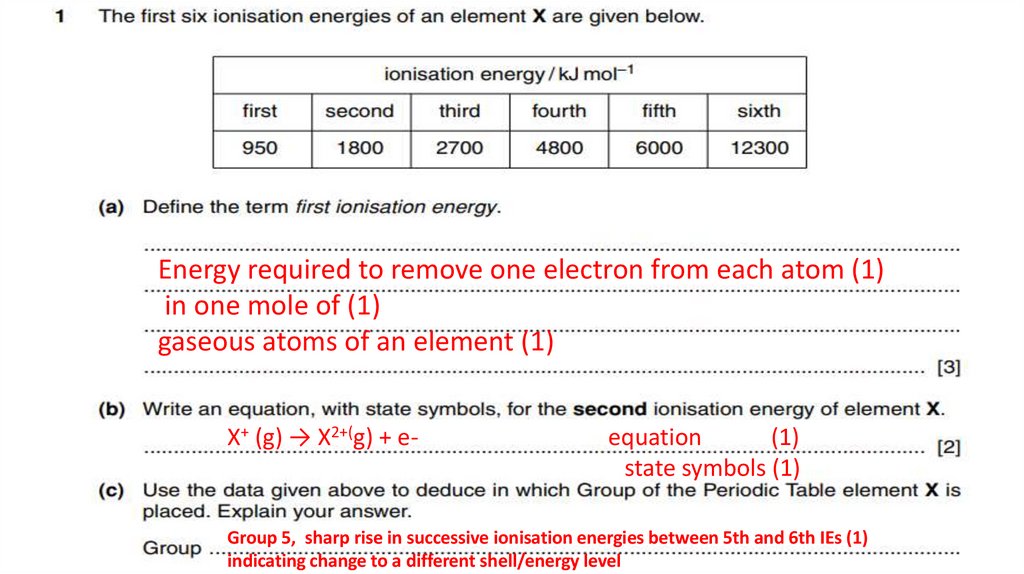
Energy required to remove one electron from each atom (1)in one mole of (1)
gaseous atoms of an element (1)
X+ (g) → X2+(g) + e-
equation
(1)
state symbols (1)
Group 5, sharp rise in successive ionisation energies between 5th and 6th IEs (1)
indicating change to a different shell/energy level








 chemistry
chemistry








