Similar presentations:
Transition metals revision 4
1.
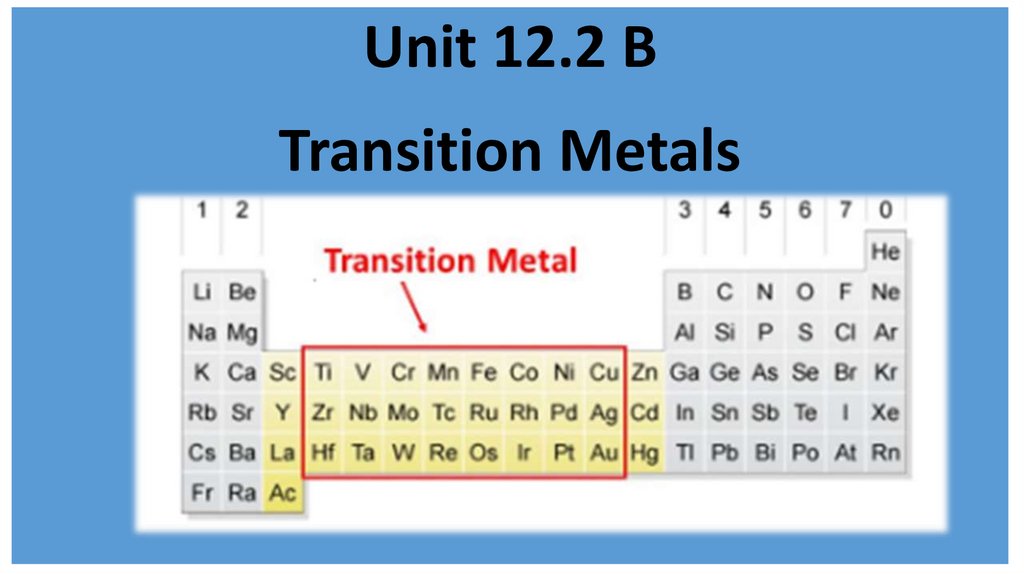
Unit 12.2 BTransition Metals
2.
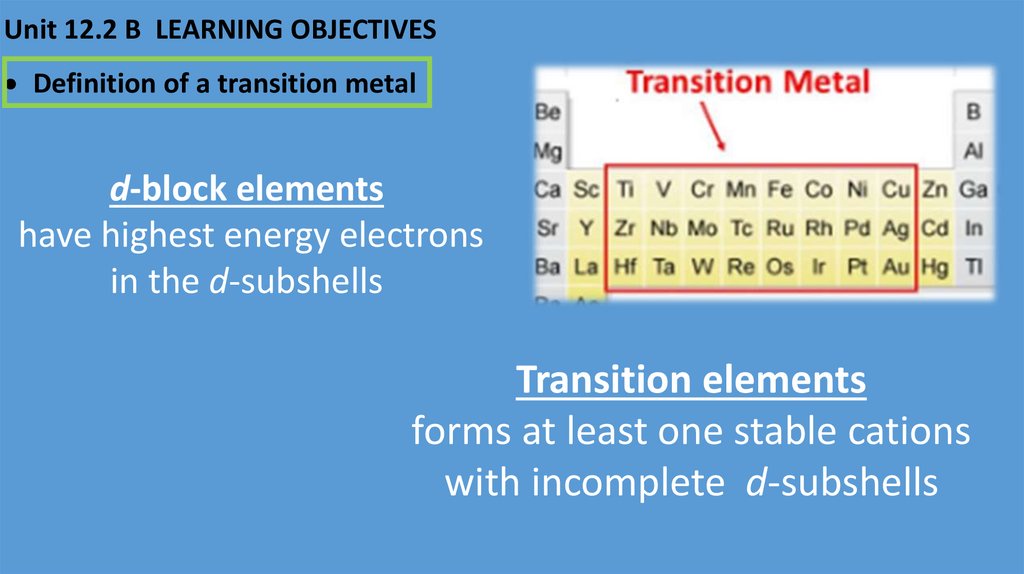
Unit 12.2 B LEARNING OBJECTIVESDefinition of a transition metal
General characteristics of transition metals
Ligands and complex formation
Shapes of transition metal complexes
Formation of coloured ions
Variability of oxidation states
Catalysis (Homogeneous and heterogeneous)
Biological applications of transition metals
3.
Unit 12.2 B LEARNING OBJECTIVESDefinition of a transition metal
d-block elements
have highest energy electrons
in the d-subshells
Transition elements
forms at least one stable cations
with incomplete d-subshells
4.
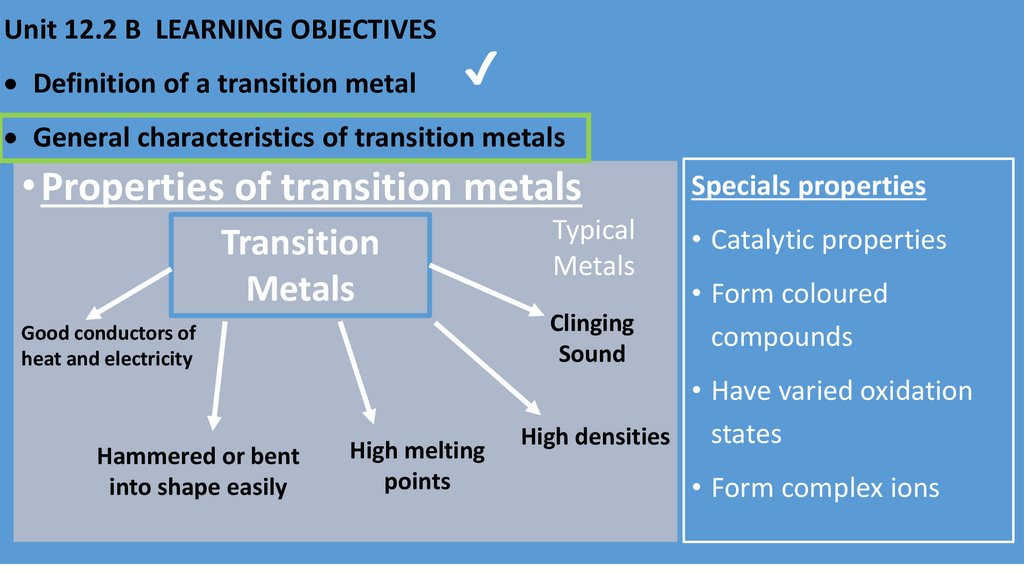
Unit 12.2 B LEARNING OBJECTIVESDefinition of a transition metal
✔
General characteristics of transition metals
• Properties of transition metals
Transition
Metals
Clinging
Sound
Good conductors of
heat and electricity
Hammered or bent
into shape easily
Typical
Metals
High melting
points
Specials properties
• Catalytic properties
• Form coloured
compounds
• Have varied oxidation
states
High densities
• Form complex ions
5.
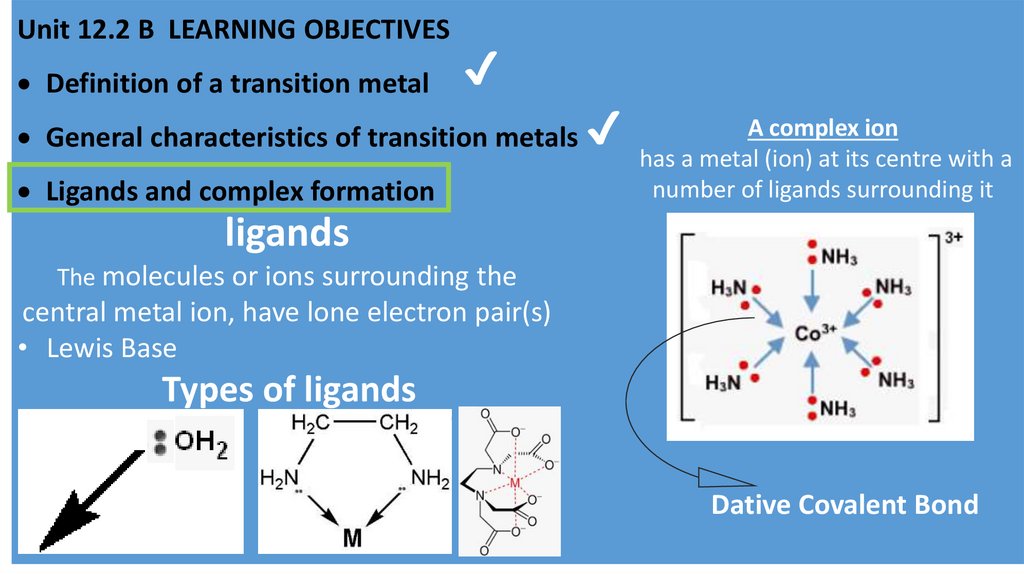
Unit 12.2 B LEARNING OBJECTIVESDefinition of a transition metal
✔
General characteristics of transition metals ✔
Ligands and complex formation
A complex ion
has a metal (ion) at its centre with a
number of ligands surrounding it
ligands
The molecules or ions surrounding the
central metal ion, have lone electron pair(s)
• Lewis Base
Types of ligands
Dative Covalent Bond
6.
Unit 12.2 B LEARNING OBJECTIVESDefinition of a transition metal
✔
General characteristics of transition metals ✔
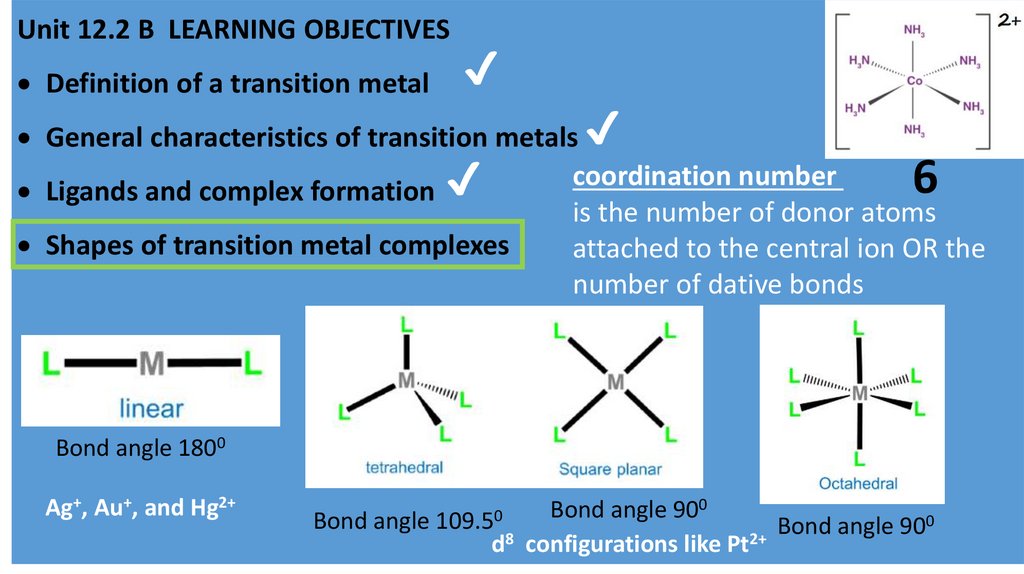
coordination number
✔
Ligands and complex formation
is the number of donor atoms
Shapes of transition metal complexes
attached to the central ion OR the
number of dative bonds
6
Bond angle 1800
Ag+, Au+, and Hg2+
Bond angle 900
0
Bond
angle
90
d8 configurations like Pt2+
Bond angle 109.50
7.
Unit 12.2 B LEARNING OBJECTIVESDefinition of a transition metal
✔
General characteristics of transition metals ✔
Ligands and complex formation ✔
Shapes of transition metal complexes ✔
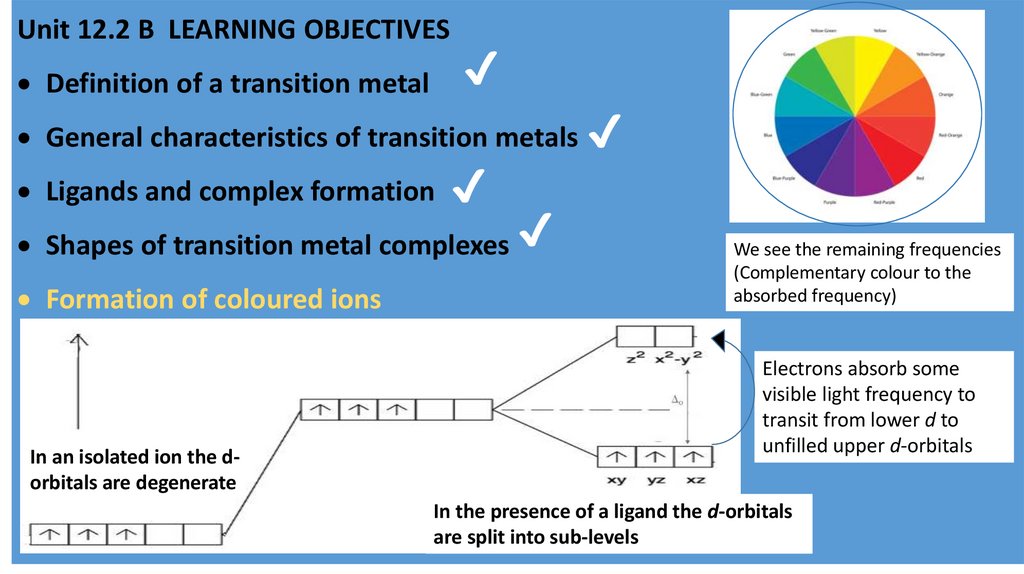
Formation of coloured ions
In an isolated ion the dorbitals are degenerate
We see the remaining frequencies
(Complementary colour to the
absorbed frequency)
Electrons absorb some
visible light frequency to
transit from lower d to
unfilled upper d-orbitals
In the presence of a ligand the d-orbitals
are split into sub-levels
8.
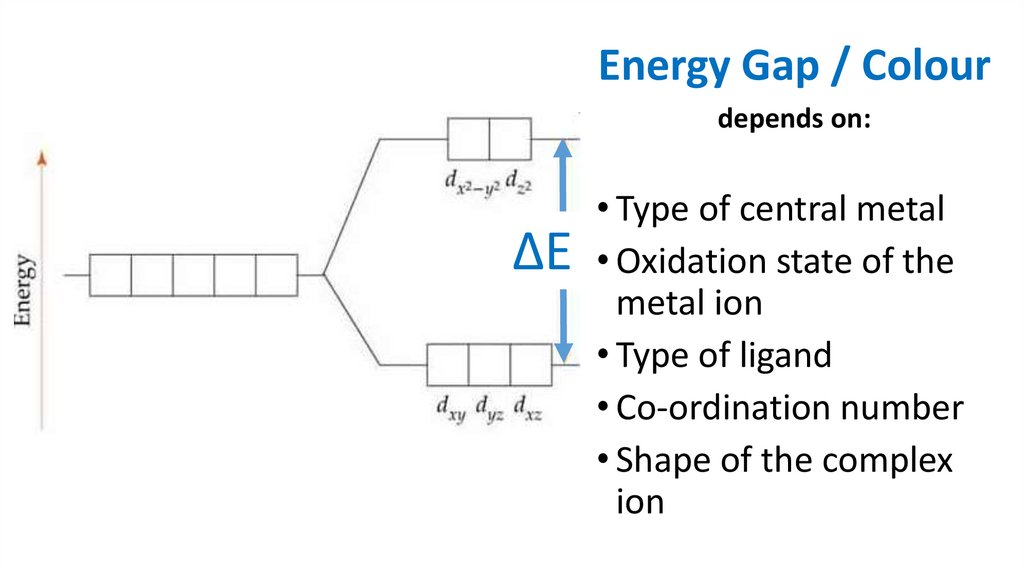
Energy Gap / Colourdepends on:
• Type of central metal
ΔE • Oxidation state of the
metal ion
• Type of ligand
• Co-ordination number
• Shape of the complex
ion
9.
Unit 12.2 B LEARNING OBJECTIVESDefinition of a transition metal
✔
General characteristics of transition metals ✔
Ligands and complex formation
✔
Shapes of transition metal complexes ✔
Formation of coloured ions
✔
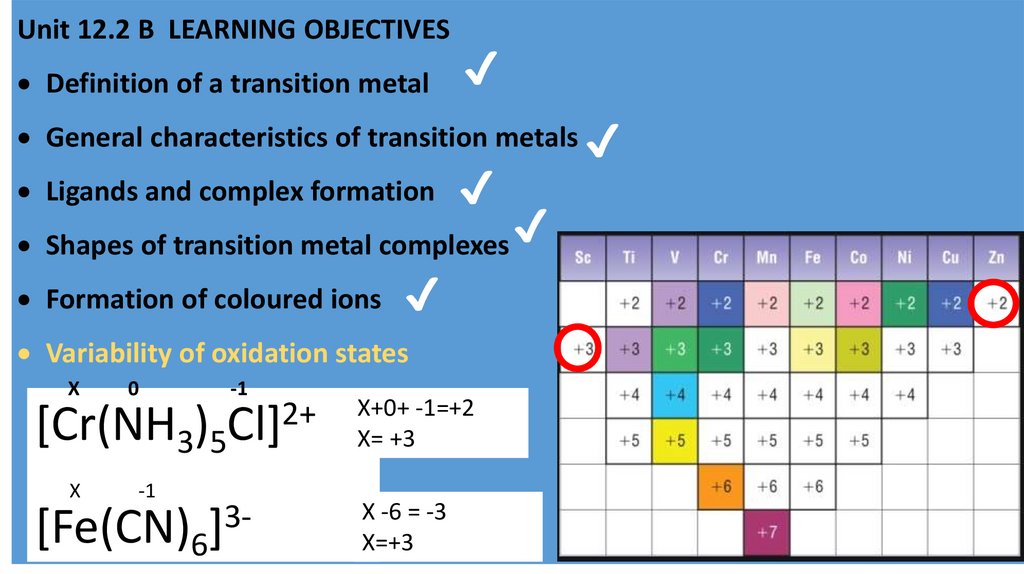
Variability of oxidation states
X
0
X
-1
-1
2+
[Cr(NH3)5Cl]
3[Fe(CN)6]
X+0+ -1=+2
X= +3
X -6 = -3
X=+3
10.
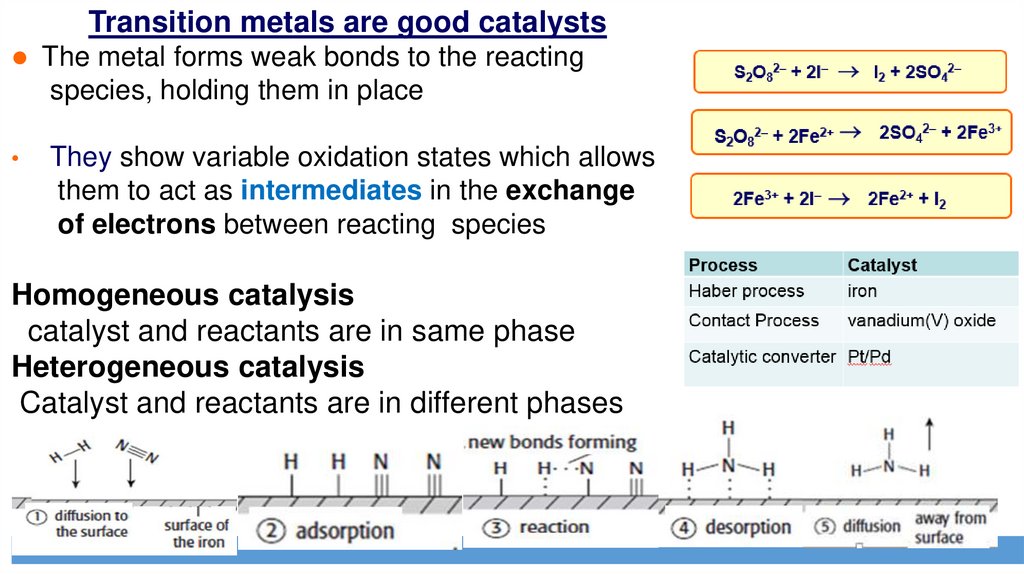
Transitionmetals are good catalysts
Unit 12.2
B OBJECTIVES
✔
The metal forms weak bonds to the reacting
Definition
of a transition
metal
species, holding
them in place
General characteristics of transition metals✔
They show variable oxidation states which allows
them toand
act complex
as intermediates
the exchange
Ligands
formationin ✔
of electrons between reacting species
✔
Shapes of transition metal complexes
Homogeneous
catalysis
Formation of coloured
ions ✔
catalyst and reactants are in same phase
Variability of oxidation
states ✔
Heterogeneous
catalysis
and
reactants areand
in heterogeneous)
different phases
Catalyst
Catalysis
(Homogeneous
11.
Unit 12.2 B LEARNING OBJECTIVESDefinition of a transition metal
✔
General characteristics of transition metals ✔
✔
Ligands and
complex
formation
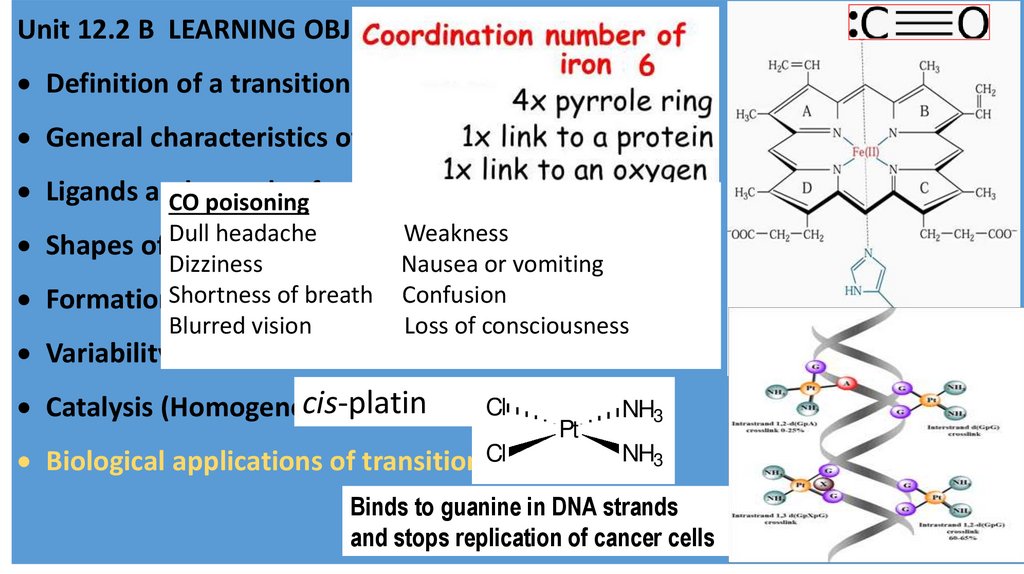
CO poisoning
headache
Weakness✔
Shapes of Dull
transition
metal complexes
Dizziness
Nausea or vomiting
of breath
FormationShortness
of coloured
ions Confusion
Blurred vision
Loss of consciousness
✔
Variability of oxidation states ✔
cis-platin
Cl
Catalysis (Homogeneous
and heterogeneous)
✔
NH3
Pt
Biological applications of transition Cl
metals
NH3
Binds to guanine in DNA strands
and stops replication of cancer cells
12.
Unit 12.2 B LEARNING OBJECTIVESDefinition of a transition metal
✔
Ligands and complex formation
✔
General characteristics of transition metals ✔
Shapes of transition metal complexes ✔
Formation of coloured ions ✔
Variability of oxidation states ✔
Catalysis (Homogeneous and heterogeneous) ✔
Biological applications of transition metals
✔