Similar presentations:
green chemistry 12 lec_72be1ad0c2e8e65a2706818895c5e1a0
1. Green Chemistry and its Role for Sustainability
2. Learning 0bjectives
To conceptualize the sustainability and ESDTo think prospectively about how to change our
education subjects to be sustainable learning tools by
Investigating examples of green chemistry applications
relevant to students
To understand the important role of the green chemistry
and how to deal with it in our practical life
3. Sustainability
• Meeting the needs of the present generation withoutcompromising the needs of future generations
Is the goal
• Green chemistry: technologies of the invention, design
and application of chemical products and processes to
reduce or to eliminate the use and generation of
hazardous substances, and where possible utilize
renewable raw materials
is the means
Primary pollution prevention not remediation
Use of chemistry for improved environmental performance
4. GREEN CHEMISTRY SUPPORTS SUSTAINABILITY BY:
• Making chemicals safe for ourhealth & environment,
• Using industrial processes that
reduce or eliminate hazardous
chemicals, &
• Designing more efficient
processes that minimize
waste
5.
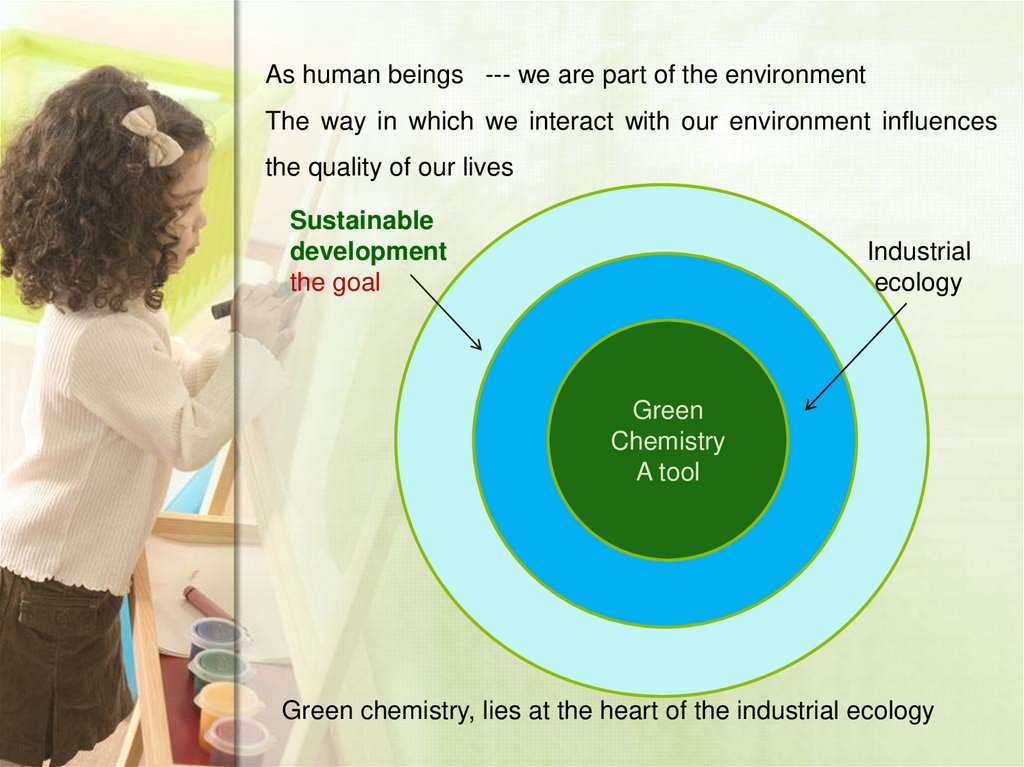
As human beings --- we are part of the environmentThe way in which we interact with our environment influences
the quality of our lives
Sustainable
development
the goal
Industrial
ecology
Green
Chemistry
A tool
Green chemistry, lies at the heart of the industrial ecology
6. GREEN CHEMISTRY MEANS…
• Preventing pollution before ithappens rather than cleaning up the
mess later.
• Saving companies money by using less energy
and fewer/safer chemicals, thus reducing costs
& impacts of pollution.
• Mitigating climate change, water & resource
depletion, & growing demands for safer food
and cleaner energy
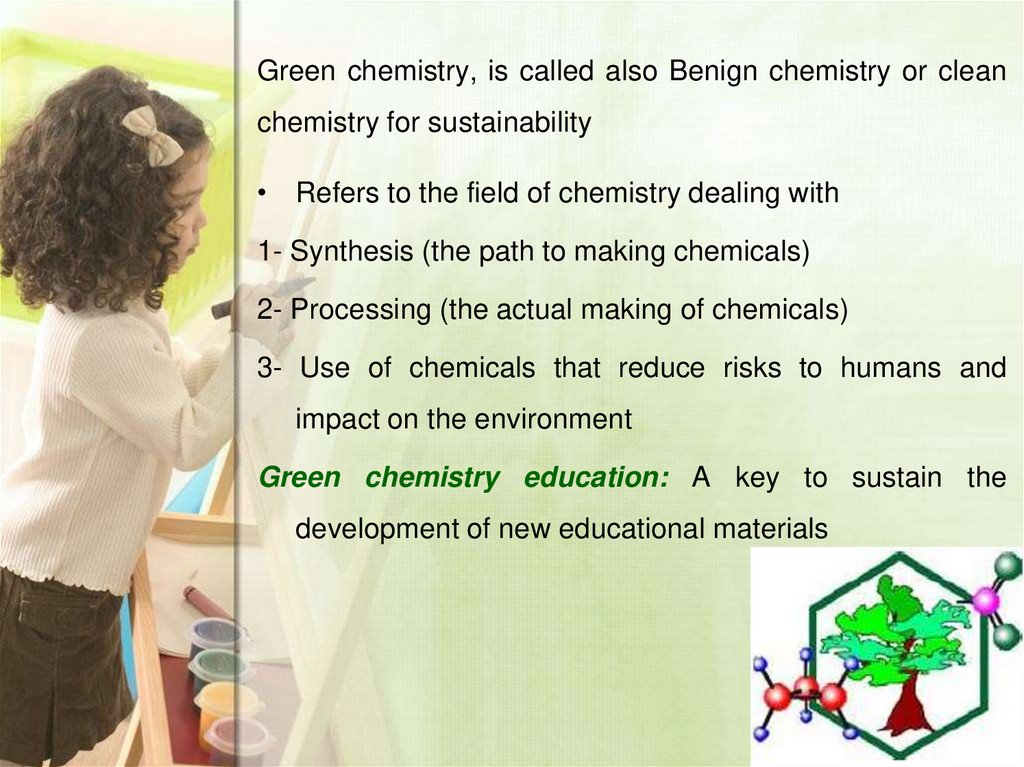
7. Green chemistry, is called also Benign chemistry or clean chemistry for sustainability
• Refers to the field of chemistry dealing with1- Synthesis (the path to making chemicals)
2- Processing (the actual making of chemicals)
3- Use of chemicals that reduce risks to humans and
impact on the environment
Green chemistry education: A key to sustain the
development of new educational materials
8. Green Chemistry Is About...
WasteMaterials
Hazard
Risk
Energy
Cost
9. Principles of Green Chemistry
Prevent waste.Design safer chemicals and products.
Design less hazardous chemical syntheses.
Use renewable feed stocks.
Use catalysts, not stoichiometric reagents: Catalysts are used in small amounts and
can carry out a single reaction many times. They are preferable to stoichiometric
reagents, which are used in excess and work only once.
Avoid chemical derivatives: Avoid using blocking or protecting groups or any
temporary modifications if possible. generate waste.
Maximize atom economy.
Use safer solvents and reaction conditions
Increase energy efficiency.
Design chemicals and products to degrade after use.
Analyze in real time to prevent pollution.
Minimize the potential for accidents.
Originally published by Paul Anastas and John Warner in Green Chemistry: Theory
and Practice (Oxford University Press: New York, 1998).
10. Green Chemistry Principles
1. Prevention: it is best to prevent pollution/waste2. Atom Economy: synthetic methods should
maximize the incorporation of all materials
used in the process into the final product
3. Less Hazardous Chemical Syntheses: synthetic
methods should use and generate non toxic
substances
11. Green Chemistry Principles 4,5,6
4. Designing Safer Chemicals: products should benontoxic & designed to effect their desired
function
5. Safer Solvents and Auxiliaries: auxiliary
substances (e.g., solvents, separation agents)
should be avoided and innocuous when used
6. Design for Energy Efficiency: Run chemical
reactions at ambient temperature and pressure
12. Green Chemistry Principles 7,8
7. Use of Renewable Feedstocks: raw material orfeedstock should be renewable rather than
depleting
8. Reduce Derivatives: Avoid unnecessary
derivatization (use of blocking groups,
protection/deprotection, temporary
modification of physical/chemical processes)
because such steps require additional reagents
and can generate waste
13. Green Chemistry Principles 9,10
9. Catalysis: Catalytic reagents (as selective aspossible) are superior to stoichiometric reagents
which are used in excess and work only once
10. Design for Degradation: Chemical products
should be designed so that at the end of their
function they break down into innocuous
degradation products
14. Green Chemistry Principles 11,12
11. Analyze in real time to prevent pollution:Include in-process real-time monitoring and
control during syntheses to minimize or
eliminate byproducts
12. Minimize accidents: Design chemicals and
their forms (solid, liquid, gas) to minimize the
potential for chemical accidents, releases,
explosions, and fires
15. Now, how can we deal with green chemistry at our practical life
Just we need to change our mind setand applying the concept in
Classrooms
laboratory
manufacture
And
finally
environment
the
surrounding
16.
• If the chemical reaction of the type• A+B
P+W
• Find alternate A or B to avoid W
• Example 1:
• Disinfection of water by chlorination. Chlorine
oxidizes the pathogens there by killing them,
but at the same time forms harmful chlorinated
compounds.
• A remedy is to use another oxidant, such as
ozone.
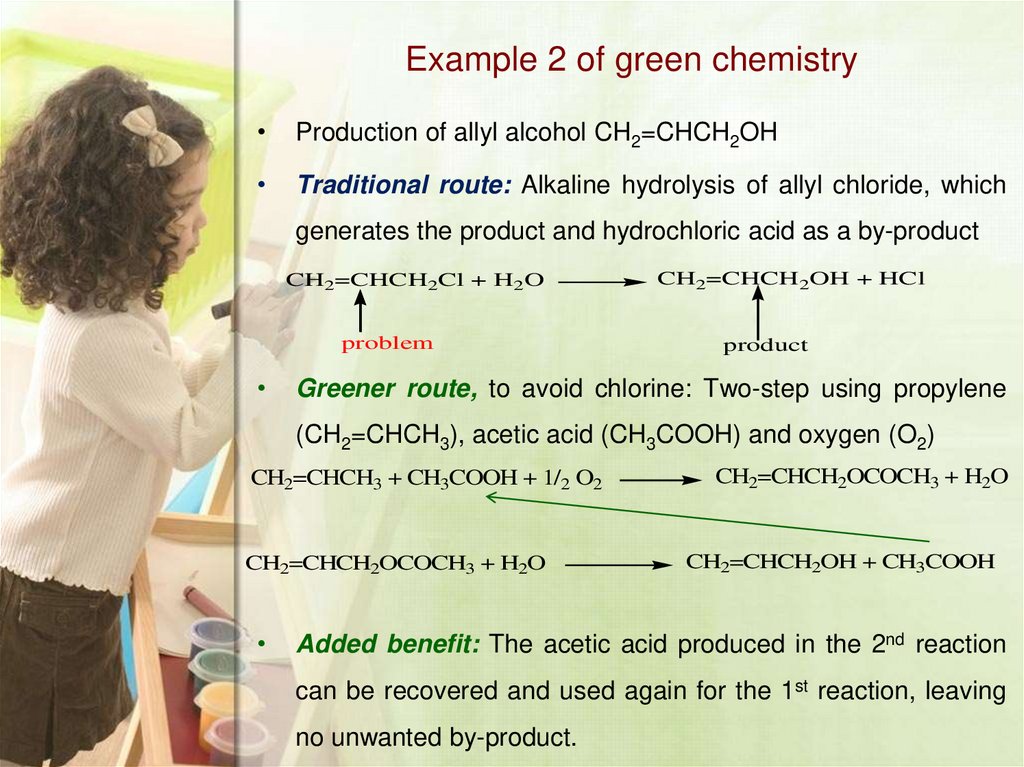
17. Example 2 of green chemistry
Production of allyl alcohol CH2=CHCH2OH
Traditional route: Alkaline hydrolysis of allyl chloride, which
generates the product and hydrochloric acid as a by-product
CH2=CHCH2Cl + H2O
problem
CH2=CHCH2OH + HCl
product
Greener route, to avoid chlorine: Two-step using propylene
(CH2=CHCH3), acetic acid (CH3COOH) and oxygen (O2)
CH2=CHCH3 + CH3COOH + 1/2 O2
CH2=CHCH2OCOCH3 + H2O
CH2=CHCH2OCOCH3 + H2O
CH2=CHCH2OH + CH3COOH
Added benefit: The acetic acid produced in the 2nd reaction
can be recovered and used again for the 1st reaction, leaving
no unwanted by-product.
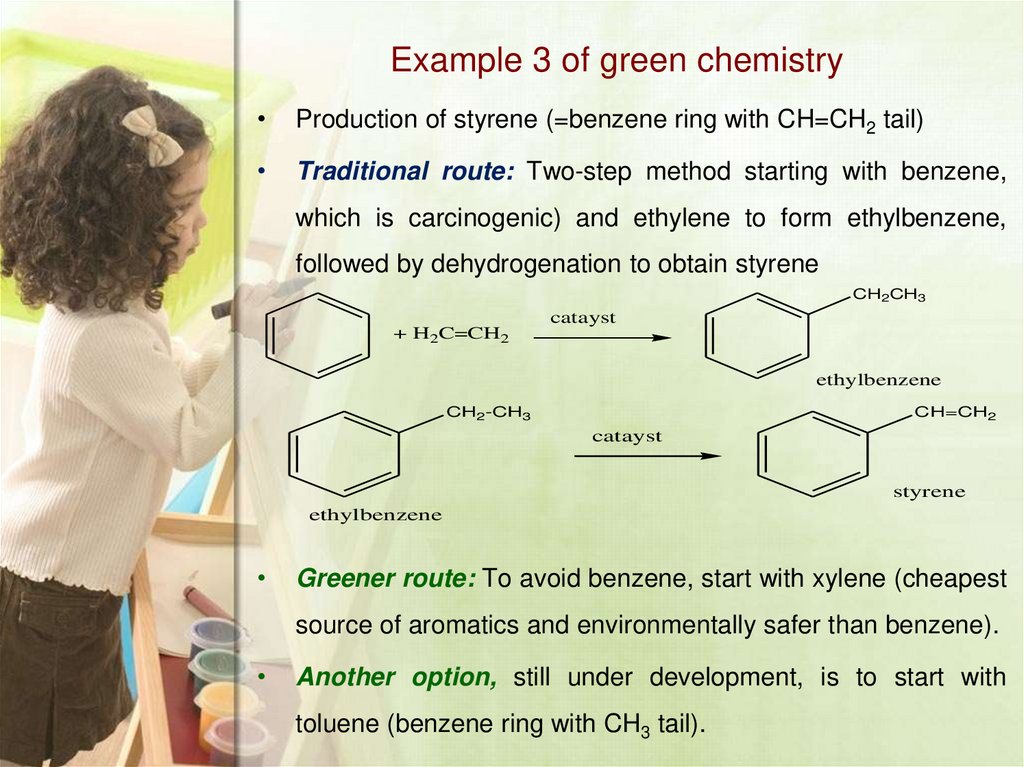
18. Example 3 of green chemistry
Production of styrene (=benzene ring with CH=CH2 tail)
Traditional route: Two-step method starting with benzene,
which is carcinogenic) and ethylene to form ethylbenzene,
followed by dehydrogenation to obtain styrene
CH2CH3
catayst
+ H2C=CH2
ethylbenzene
CH2-CH3
CH=CH2
catayst
styrene
ethylbenzene
Greener route: To avoid benzene, start with xylene (cheapest
source of aromatics and environmentally safer than benzene).
Another option, still under development, is to start with
toluene (benzene ring with CH3 tail).
19. Green chemistry education
Chemistry students need to be encouraged toconsider the principles of green chemistry when
designing processes and choosing reagents
Interactive Teaching Units (ITU) have been
developed specifically to introduce undergraduate
students to green chemistry
There are numerous scholarships and grants
available for researchers and young scholars who
are furthering the goals of green chemistry
20. conclusion
Green Chemistry:Preventing Pollution
Sustaining the Earth
Green chemistry has come a long way since its birth in 1991,
growing from a small grassroots idea into a new approach to
scientifically-based environmental protection
All over the world, governments and industries are working
with “green” chemists to transform the economy into a
sustainable enterprise
Who knows? Green chemistry may be the next social
movement that will set aside all the world’s differences and
allow for the creation of an environmentally commendable
civilization
21. References
http://www.epa.gov/greenchemistry/http://www.epa.gov/greenchemistry/pubs/educat.html
http://www.epa.gov/greenchemistry/
http://www.epa.gov/greenchemistry/pubs/principles.html
http://en.wikipedia.org/wiki/Green_chemistry
http://portal.acs.org/portal/acs/corg/content?_nfpb=true&_pageLabel=PP_TRANSITIO
NMAIN&node_id=830&use_sec=false&sec_url_var=region1&__uuid=76247a16-94d0458e-9092-10de1c35f2c6
http://books.google.com/books?id=ZMjkTMwO3NkC&dq=green+chemistry&printsec=fr
ontcover&source=bl&ots=ZdGD63CxOJ&sig=vM94PxekSEhIX3a9yFOPpDAOXGo&hl=
en&ei=mD9RSqSoDqDMjAfJg4mfBQ&sa=X&oi=book_result&ct=result&resnum=8
http://books.google.com/books?id=ZMjkTMwO3NkC&dq=green+chemistry&printsec=fr
ontcover&source=bl&ots=ZdGD63CxOJ&sig=vM94PxekSEhIX3a9yFOPpDAOXGo&hl=
en&ei=mD9RSqSoDqDMjAfJg4mfBQ&sa=X&oi=book_result&ct=result&resnum=8
2009
zenab_77@mans.edu.eg