Similar presentations:
Solutions
1.
SOLUTIONS2.
In chemistry, a solution is a homogeneousmixture composed of only one phase. In such a
mixture, a solute is dissolved in another
substance, known as a solvent.
The ability of one
compound to dissolve in
another compound is called
solubility.
3.
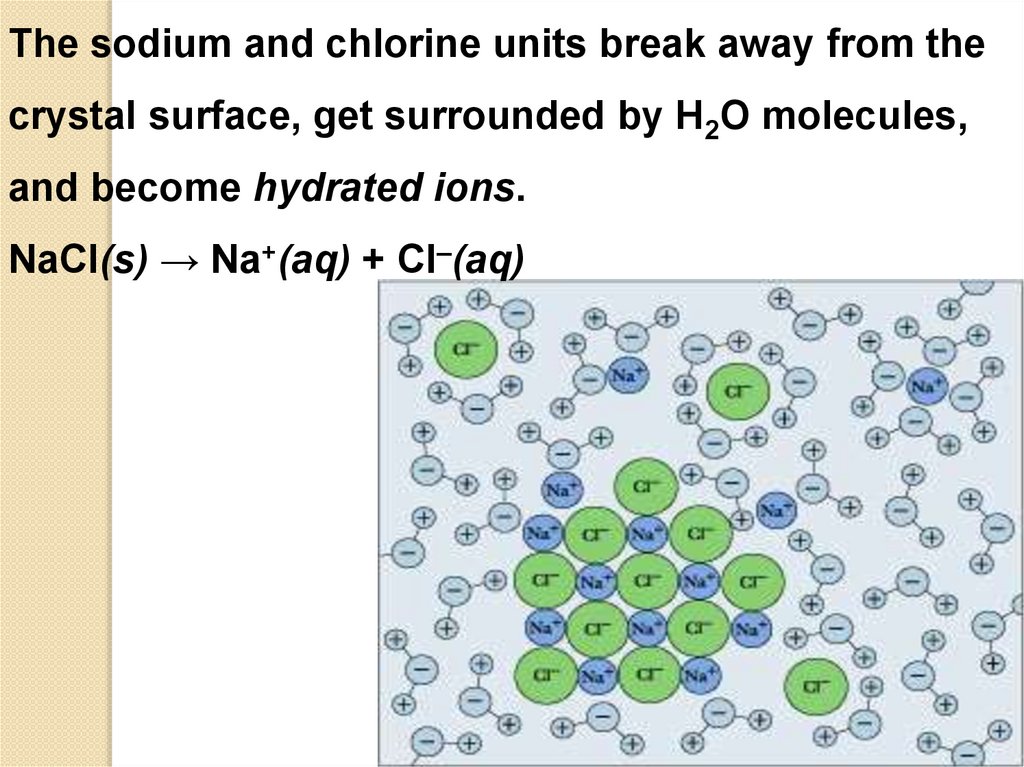
The sodium and chlorine units break away from thecrystal surface, get surrounded by H2O molecules,
and become hydrated ions.
NaCl(s) → Na+(aq) + Cl–(aq)
4.
solubility of gases.In physics, Henry's law is one of the gas laws
formulated by William Henry in 1803.
At a constant temperature, the
amount of a given gas that dissolves
in a given type and volume of liquid is
directly proportional to the partial
pressure of that gas in equilibrium
with that liquid.
p= KH C
5.
where p is the partial pressure of thesolute in the gas above the solution,
c is the concentration of the solute and
kH (Henry's constant ) is a constant with
the dimensions of pressure divided by
concentration.
6.
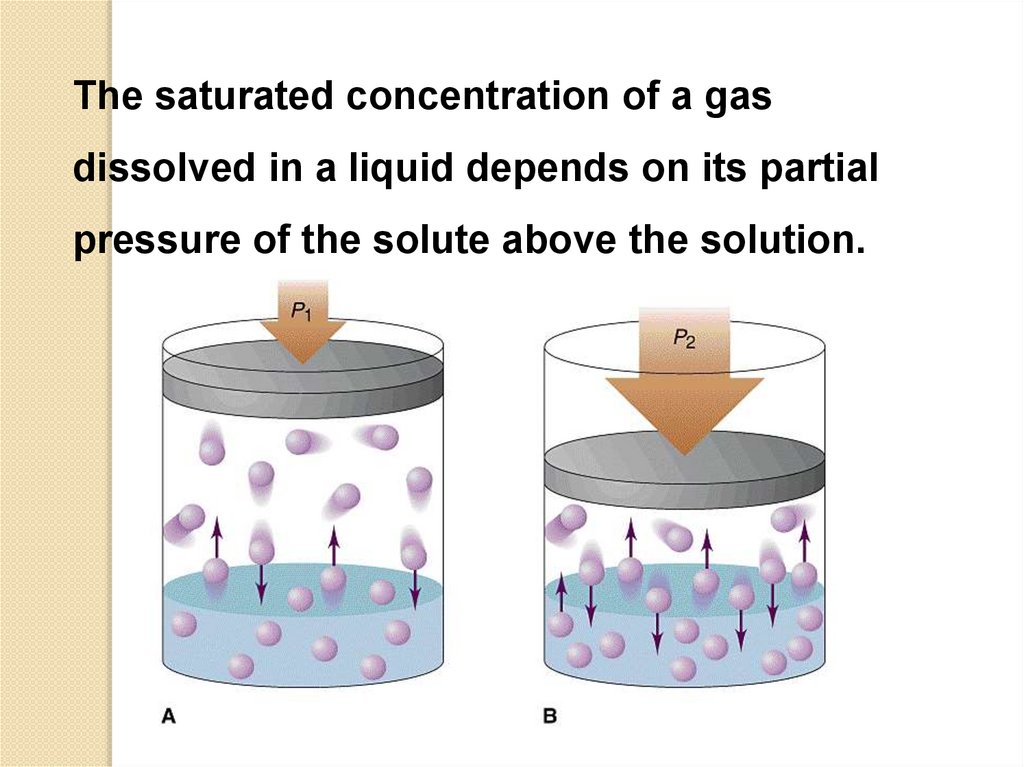
The saturated concentration of a gasdissolved in a liquid depends on its partial
pressure of the solute above the solution.
7.
Henry's law applications.•To increase the solubiity of CO2 in soft drinks and soda
water, the bottle is sealed under high pressure.
•To minimise the painful effects accompanying the
decompression of deep sea divers, oxygen diluted with
less soluble helium gas is used as breathing gas.
•In lungs where oxygen is present in air with high partial
pressure, haemoglobin combines with oxygen to form
oxyhaemoglobin. In tissues where partial pressure of
oxygen is low, oxyhaemoglobin releases oxygen for
utilization in cellular activities.
8.
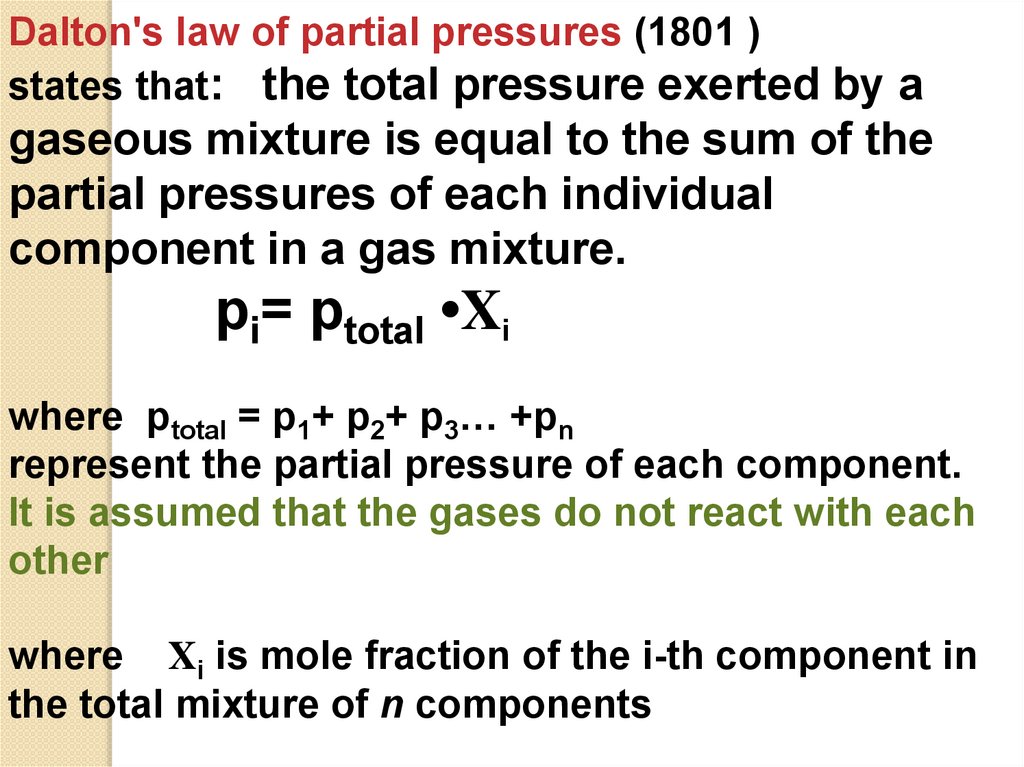
Dalton's law of partial pressures (1801 )states that: the total pressure exerted by a
gaseous mixture is equal to the sum of the
partial pressures of each individual
component in a gas mixture.
pi= ptotal •Xi
where ptotal = p1+ p2+ p3… +pn
represent the partial pressure of each component.
It is assumed that the gases do not react with each
other
where Xi is mole fraction of the i-th component in
the total mixture of n components
9.
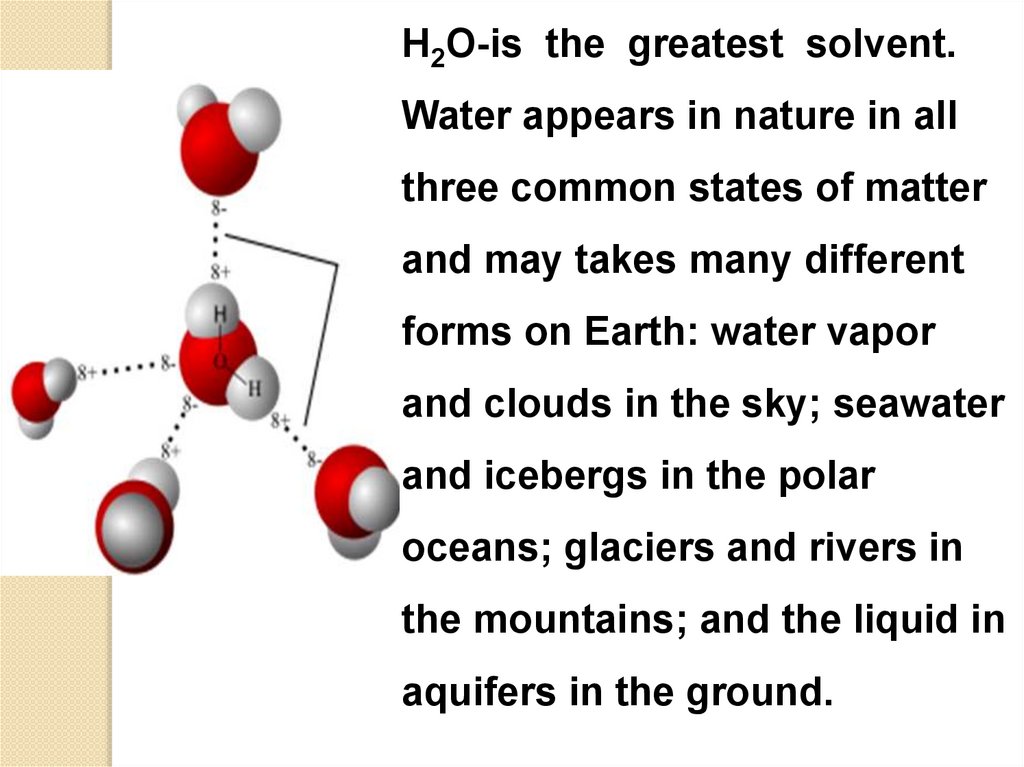
H2O-is the greatest solvent.Water appears in nature in all
three common states of matter
and may takes many different
forms on Earth: water vapor
and clouds in the sky; seawater
and icebergs in the polar
oceans; glaciers and rivers in
the mountains; and the liquid in
aquifers in the ground.
10.
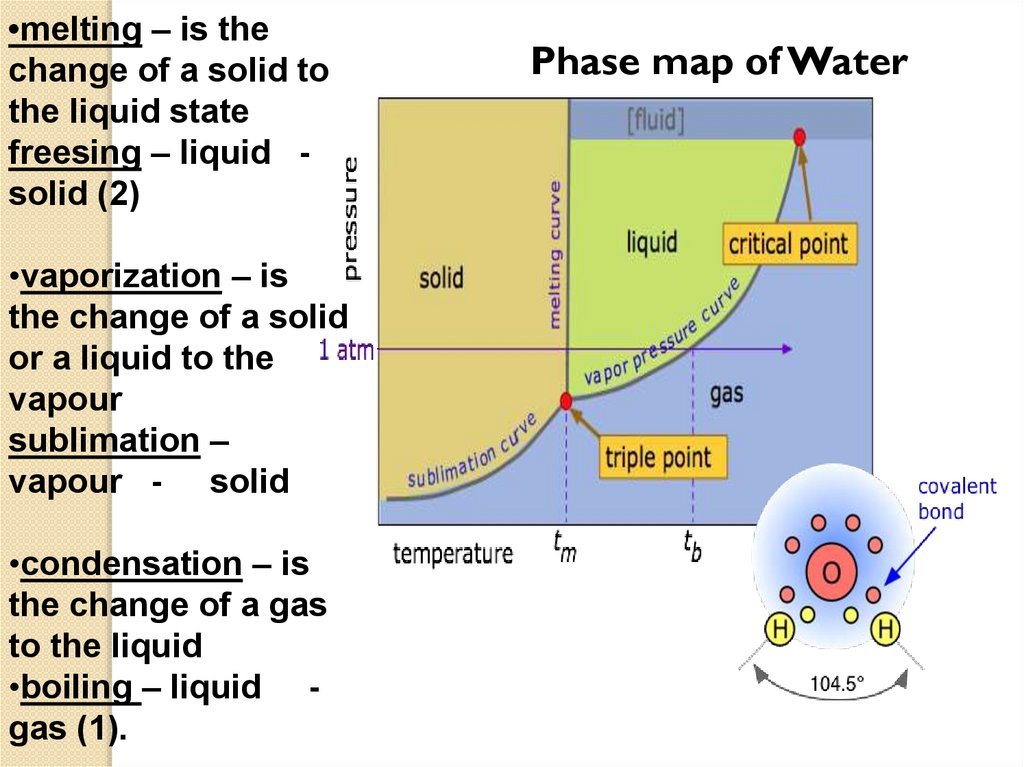
•melting – is thechange of a solid to
the liquid state
freesing – liquid solid (2)
•vaporization – is
the change of a solid
or a liquid to the
vapour
sublimation –
vapour - solid
•condensation – is
the change of a gas
to the liquid
•boiling – liquid gas (1).
Phase map of Water
11.
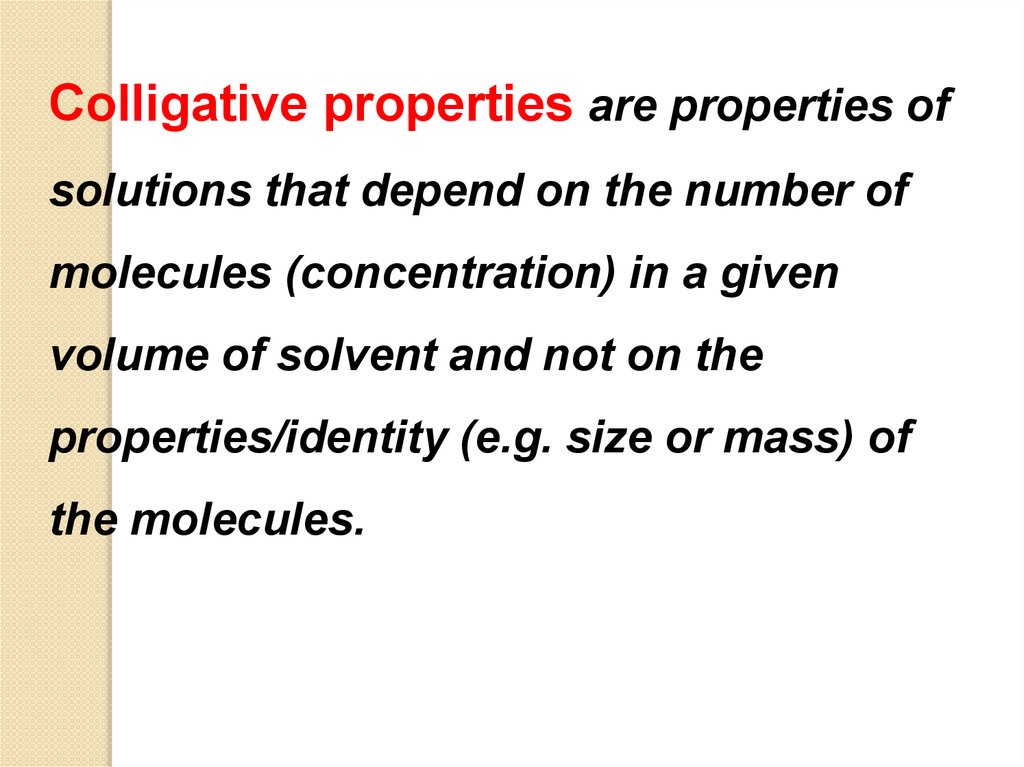
Colligative properties are properties ofsolutions that depend on the number of
molecules (concentration) in a given
volume of solvent and not on the
properties/identity (e.g. size or mass) of
the molecules.
12.
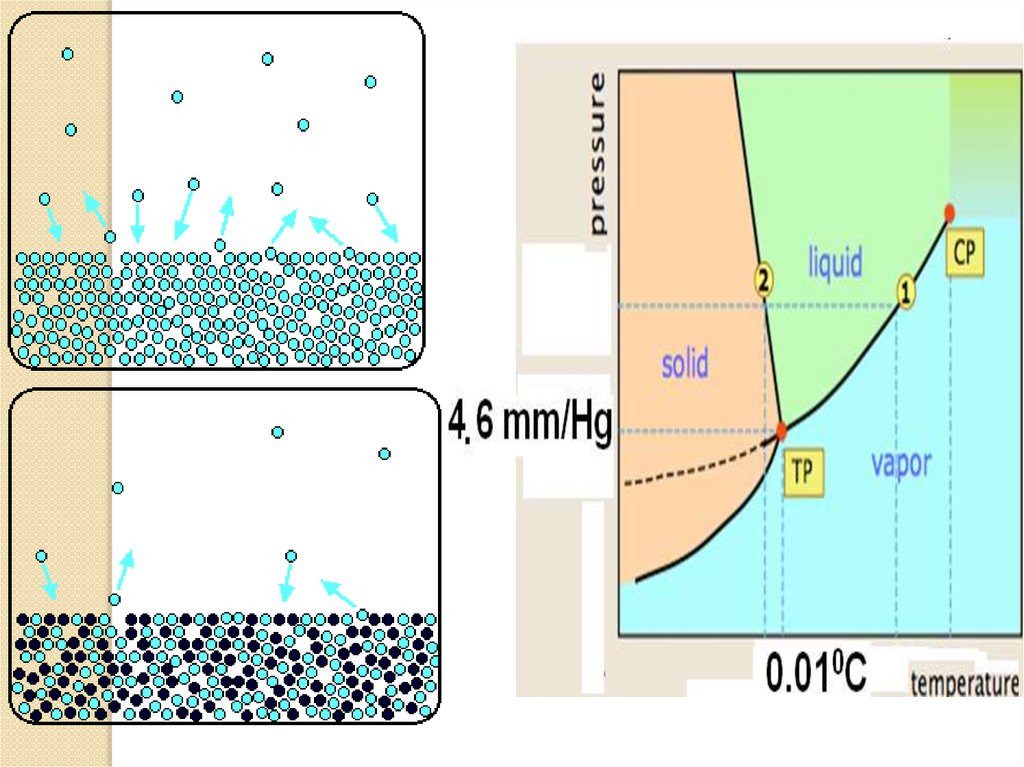
1. Vapor pressure (∆P) ofsolutions: Raoult's law.
Vapor pressure or equilibrium vapor
pressure is the pressure of a vapor in
thermodynamic equilibrium with its condensed
phases in a closed container. All liquids and solids have
a tendency to evaporate into a gaseous form, and all
gases have a tendency to condense back to their
liquid or solid form.
13.
14.
15.
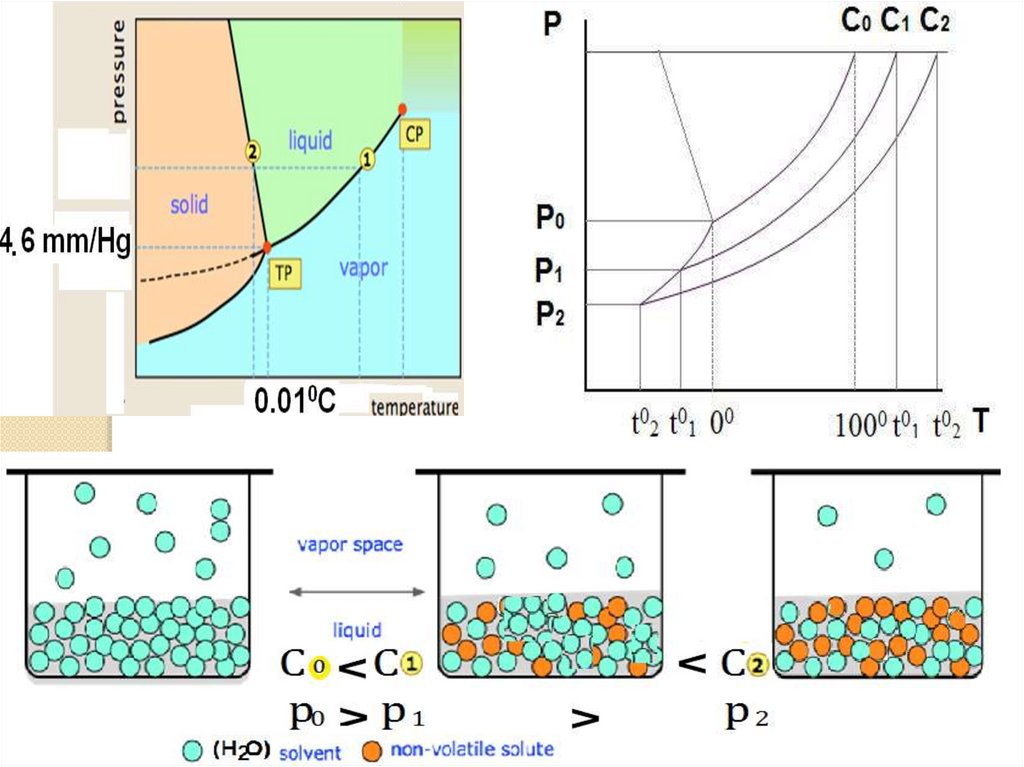
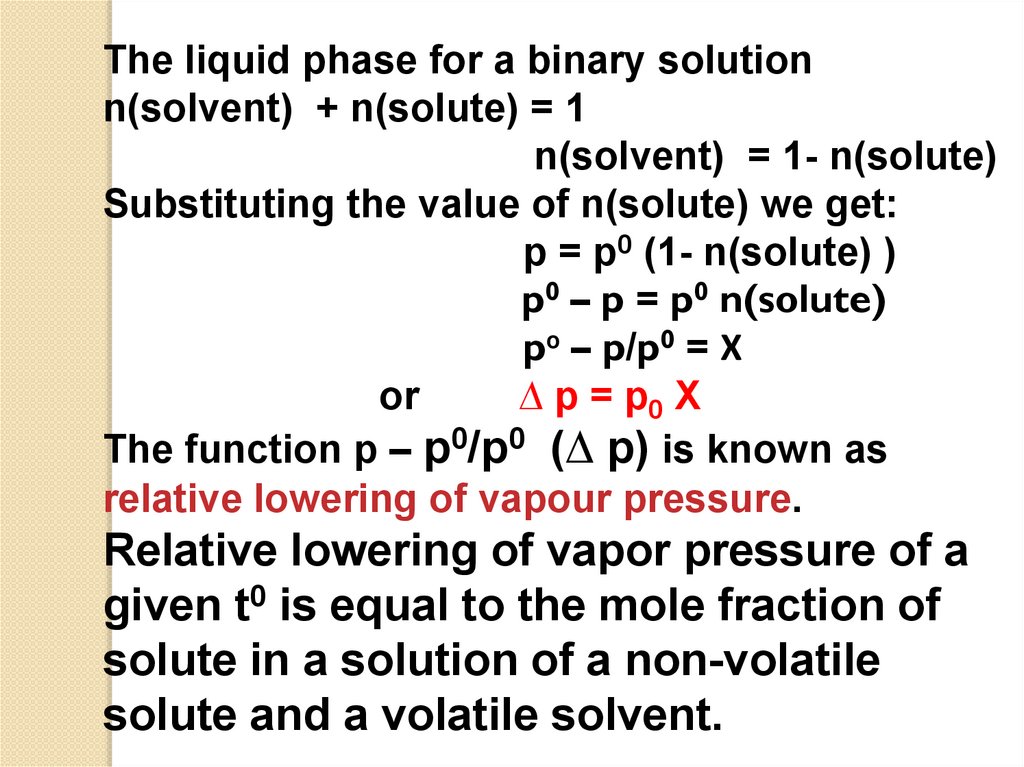
The liquid phase for a binary solutionn(solvent) + n(solute) = 1
n(solvent) = 1- n(solute)
Substituting the value of n(solute) we get:
p = p0 (1- n(solute) )
p0 – p = p0 n(solute)
p0 – p/p0 = Χ
or
∆ p = p0 Χ
The function p – p0/p0 (∆ p) is known as
relative lowering of vapour pressure.
Relative lowering of vapor pressure of a
given t0 is equal to the mole fraction of
solute in a solution of a non-volatile
solute and a volatile solvent.
16.
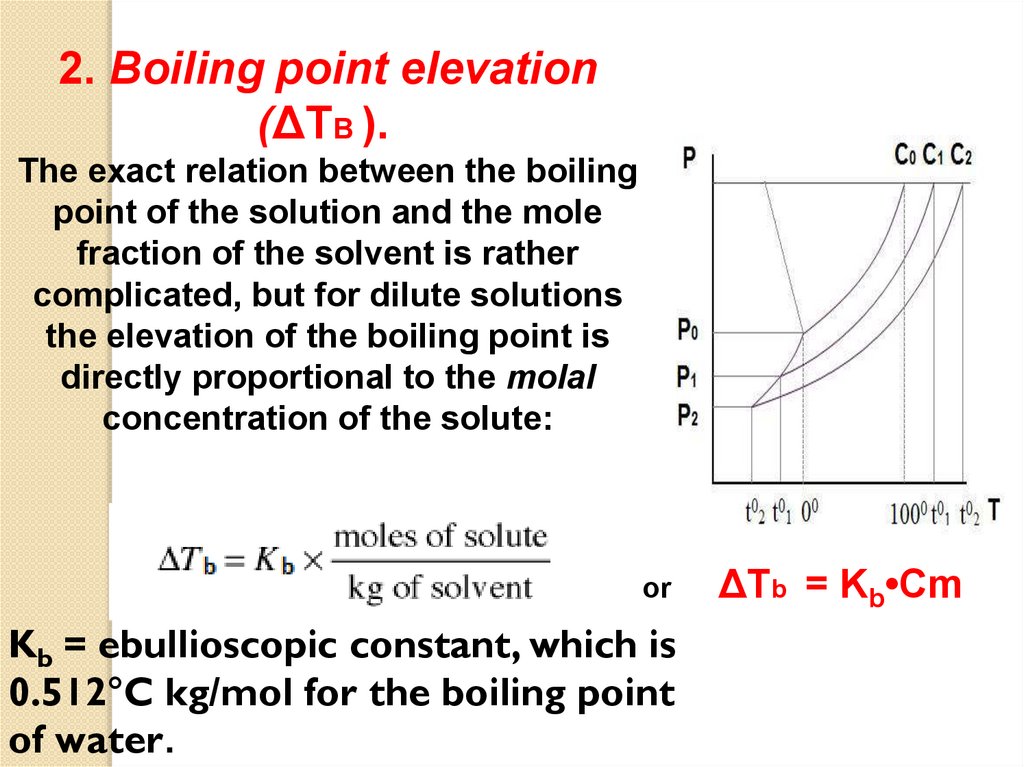
2. Boiling point elevation(ΔTB ).
The exact relation between the boiling
point of the solution and the mole
fraction of the solvent is rather
complicated, but for dilute solutions
the elevation of the boiling point is
directly proportional to the molal
concentration of the solute:
or
Kb = ebullioscopic constant, which is
0.512°C kg/mol for the boiling point
of water.
ΔTb = Kb•Cm
17.
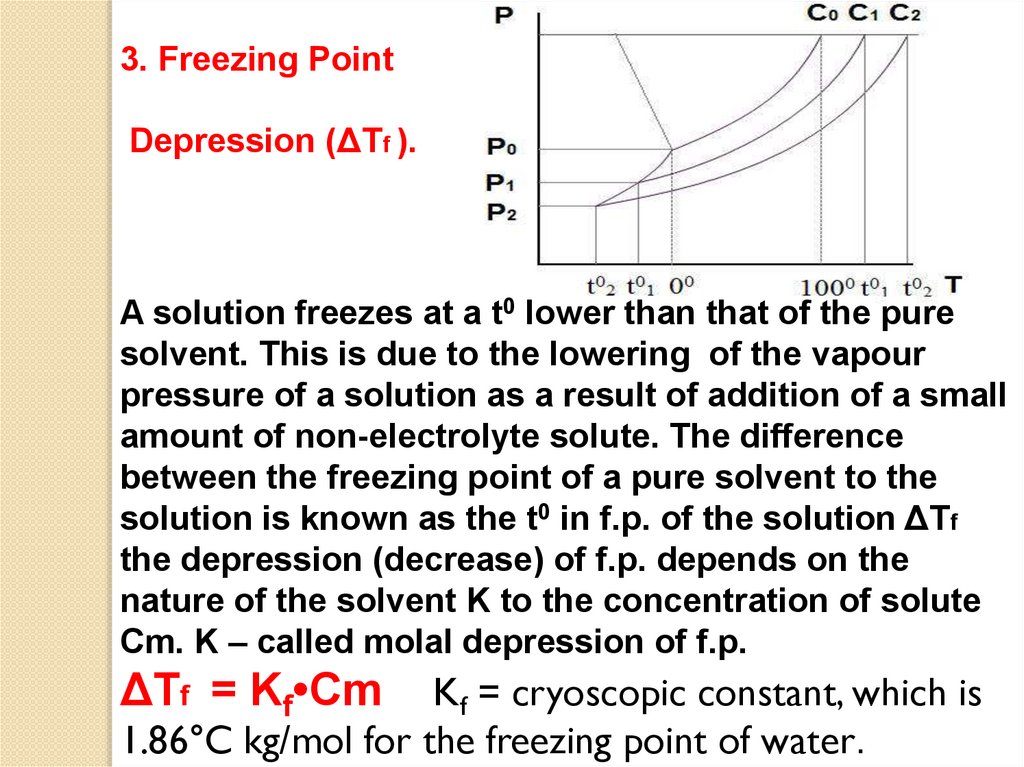
3. Freezing PointDepression (ΔTf ).
A solution freezes at a t0 lower than that of the pure
solvent. This is due to the lowering of the vapour
pressure of a solution as a result of addition of a small
amount of non-electrolyte solute. The difference
between the freezing point of a pure solvent to the
solution is known as the t0 in f.p. of the solution ΔTf
the depression (decrease) of f.p. depends on the
nature of the solvent K to the concentration of solute
Cm. K – called molal depression of f.p.
ΔTf = Kf•Cm
Kf = cryoscopic constant, which is
1.86°C kg/mol for the freezing point of water.
18.
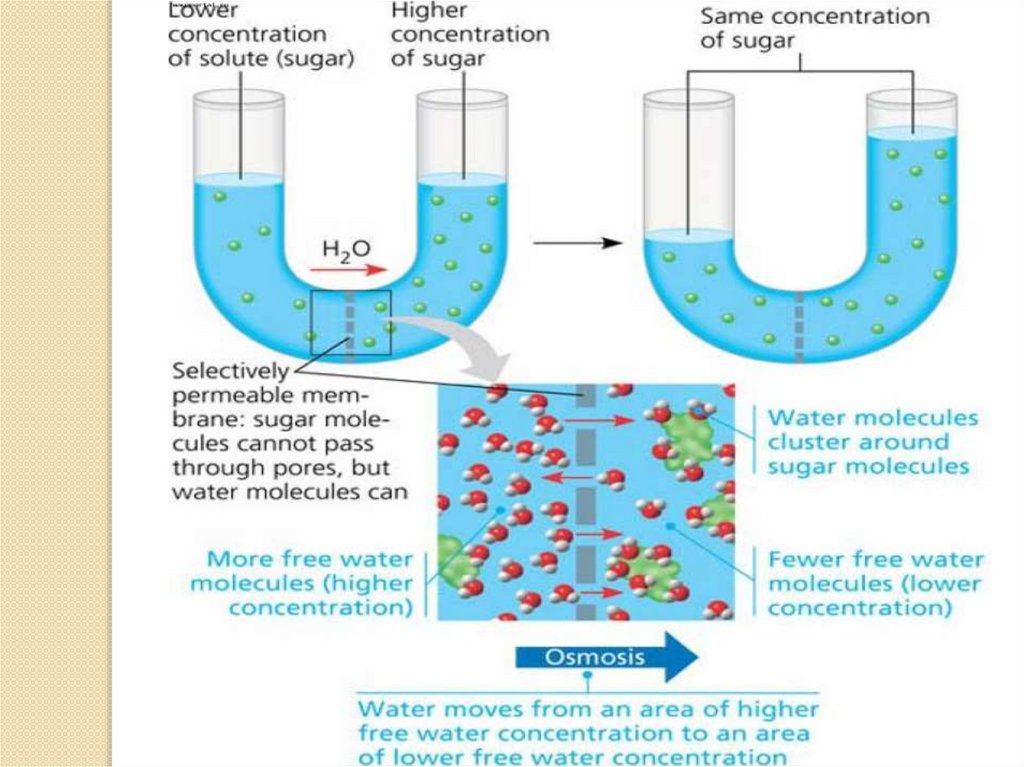
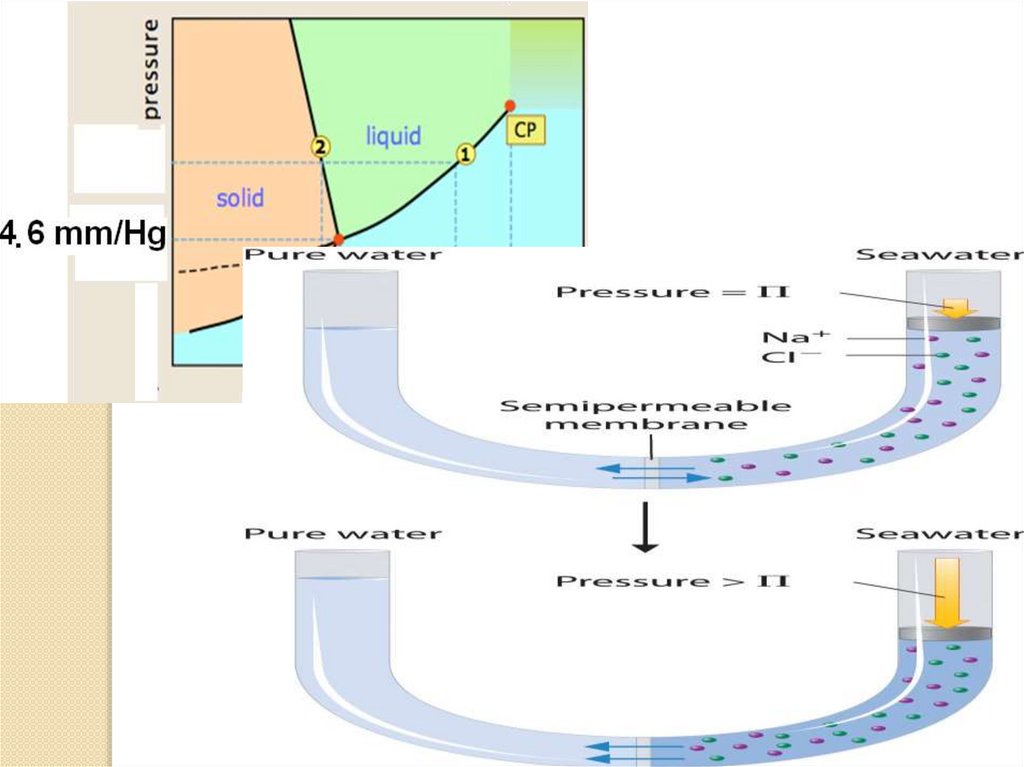
4. Osmosis is the movement of solventmolecules through a selectively permeable
membrane into a region of higher solute
concentration, aiming to equalize the solute
concentrations on the two sides. It may also
be used to describe a physical process in
which any solvent moves, without input of
energy,across a semipermeable membrane
(permeable to the solvent, but not the solute)
separating two solutions of different
concentrations. Although osmosis does not
require input of energy, it does use kinetic
energy and can be made to do work.
19.
20.
Net movement of solvent is from the lessconcentrated (hypotonic) to the moreconcentrated (hypertonic) solution, whichtends to reduce the difference in
concentrations. This effect can be countered
by increasing the pressure of the hypertonic
solution, with respect to the hypotonic.
21.
The osmotic pressure is defined to be thepressure required to maintain an equilibrium,
with no net movement of solvent. Osmotic
pressure is a colligative property, meaning that
the osmotic pressure depends on the molar
concentration of the solute but not on its
identity.
22.
Osmotic pressure (π) is the pressure which needs tobe applied to a solution to prevent the inward flow of
water across a semipermeable membrane
π= CRT.
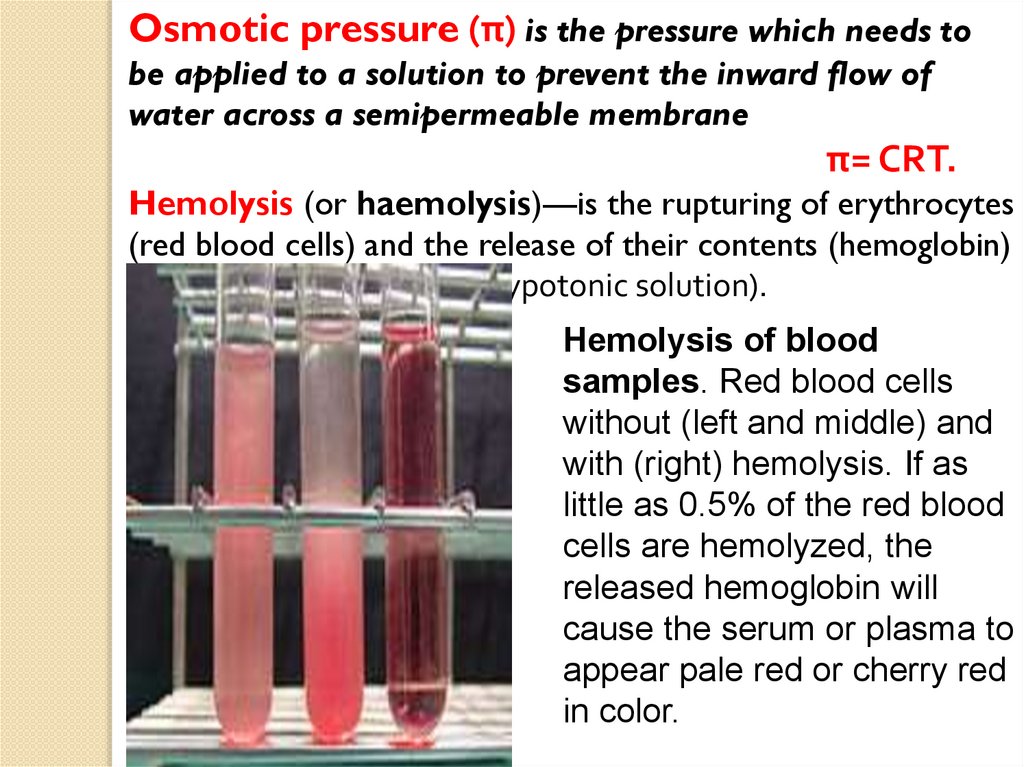
Hemolysis (or haemolysis)—is the rupturing of erythrocytes
(red blood cells) and the release of their contents (hemoglobin)
into surrounding fluid (in hypotonic solution).
Hemolysis of blood
samples. Red blood cells
without (left and middle) and
with (right) hemolysis. If as
little as 0.5% of the red blood
cells are hemolyzed, the
released hemoglobin will
cause the serum or plasma to
appear pale red or cherry red
in color.
23.
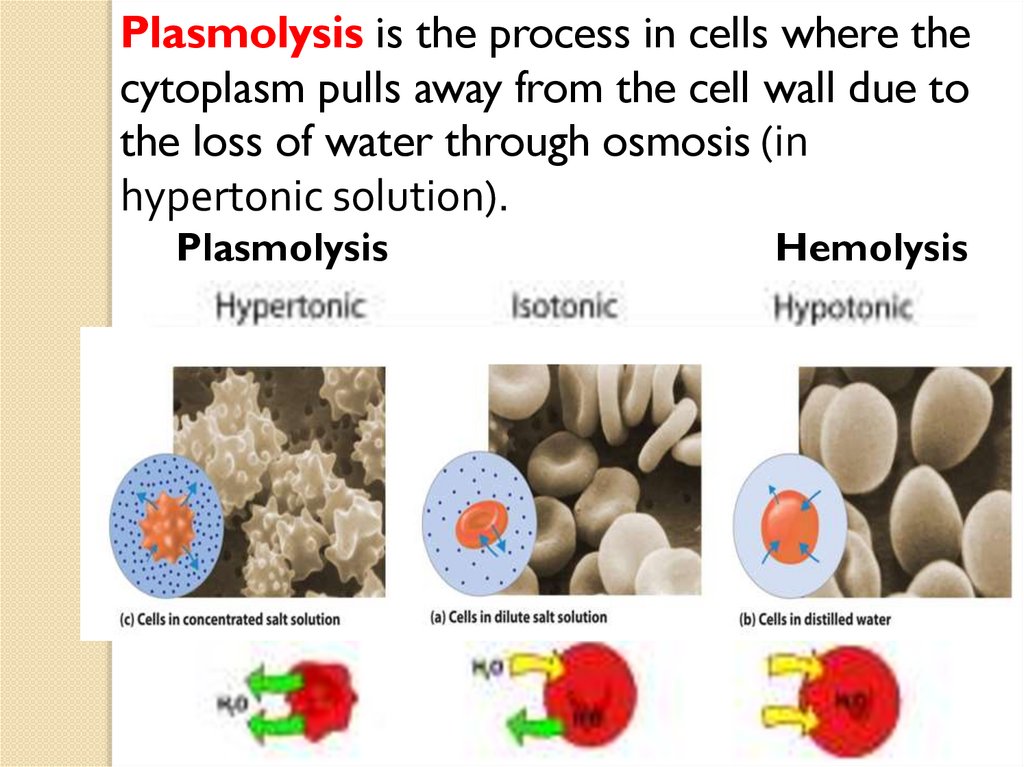
Plasmolysis is the process in cells where thecytoplasm pulls away from the cell wall due to
the loss of water through osmosis (in
hypertonic solution).
Plasmolysis
Hemolysis
24.
The colligative properties of NON- VOLATILE solutions:1. Vapor pressure (∆P) of solutions
∆p = p0Χ
2. Boiling point elevation (ΔTB )
ΔTb = K•Cm
3. The Freezing Point Depression (ΔTf ) ΔTf = K•Cm
4. Osmotic pressure (π)
π= CRT
25.
An electrolyte is any substance containing free ionsthat make the substance electrically conductive. The
most typical electrolyte is an ionic solution, but
molten electrolytes and solid electrolytes are also
possible.
Commonly, electrolytes are solutions of acids, bases
or salts.
26.
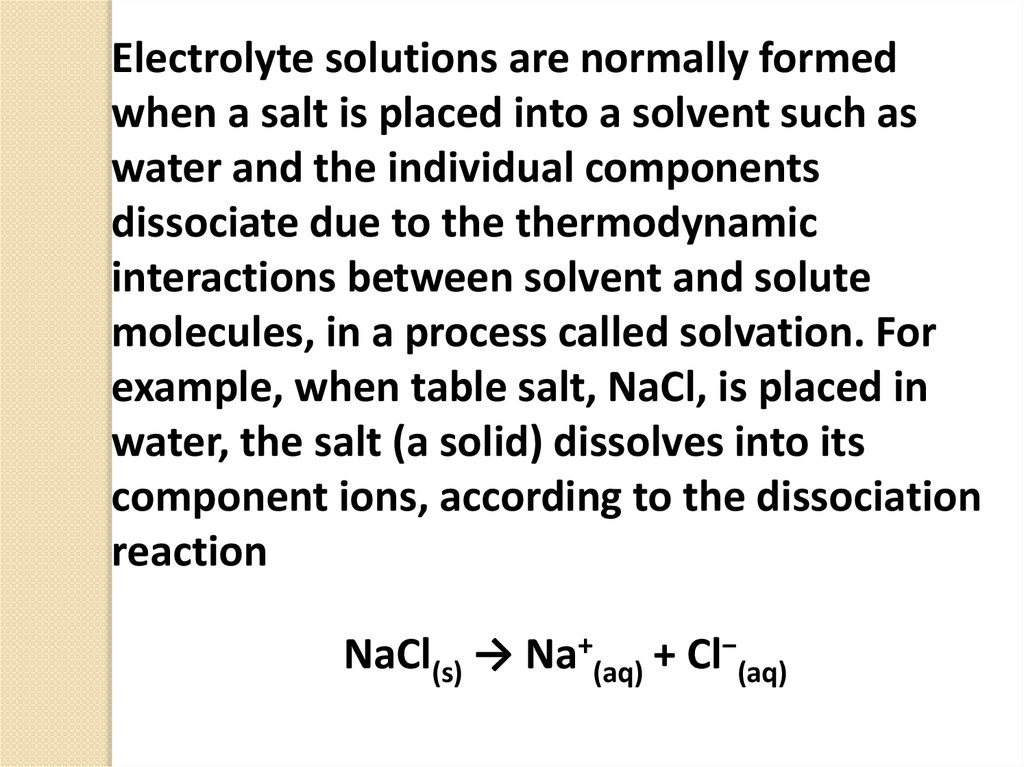
Electrolyte solutions are normally formedwhen a salt is placed into a solvent such as
water and the individual components
dissociate due to the thermodynamic
interactions between solvent and solute
molecules, in a process called solvation. For
example, when table salt, NaCl, is placed in
water, the salt (a solid) dissolves into its
component ions, according to the dissociation
reaction
NaCl(s) → Na+(aq) + Cl−(aq)
27.
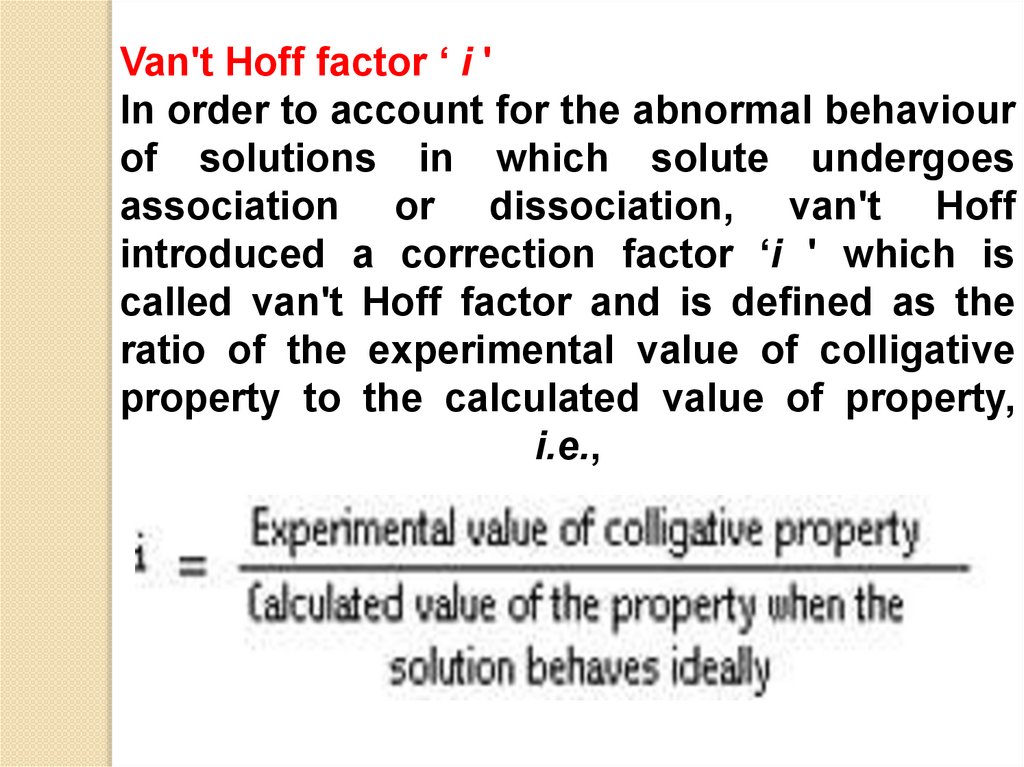
Van't Hoff factor ‘ i 'In order to account for the abnormal behaviour
of solutions in which solute undergoes
association or dissociation, van't Hoff
introduced a correction factor ‘i ' which is
called van't Hoff factor and is defined as the
ratio of the experimental value of colligative
property to the calculated value of property,
i.e.,
28.
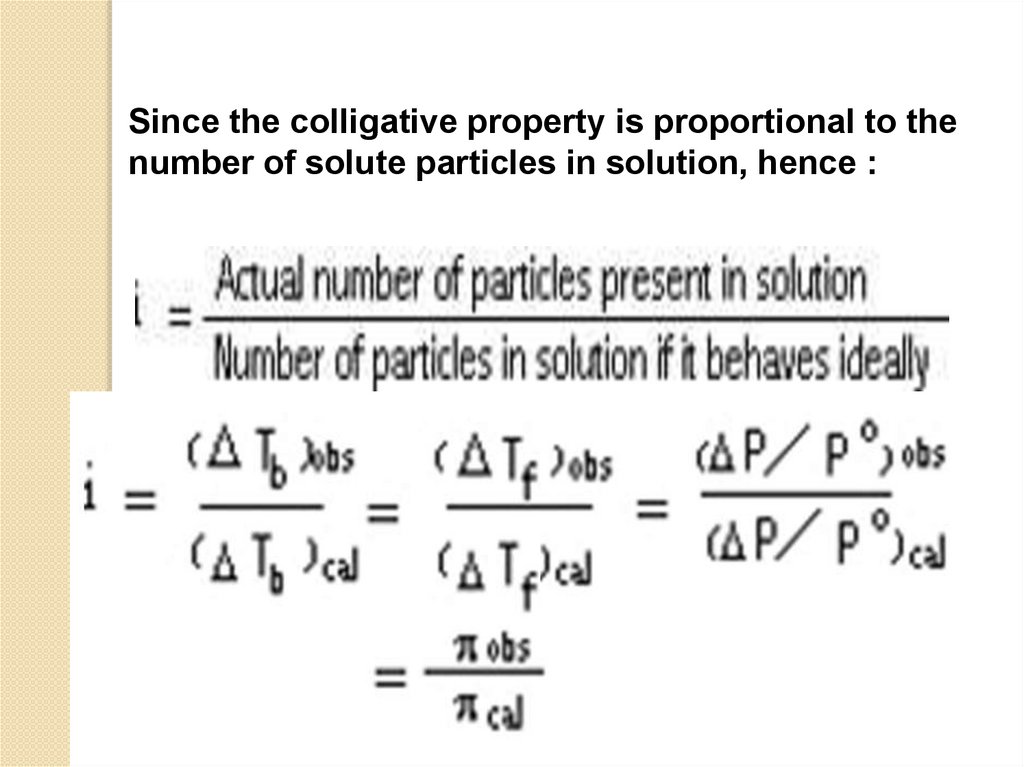
Since the colligative property is proportional to thenumber of solute particles in solution, hence :
or we may write :
29.
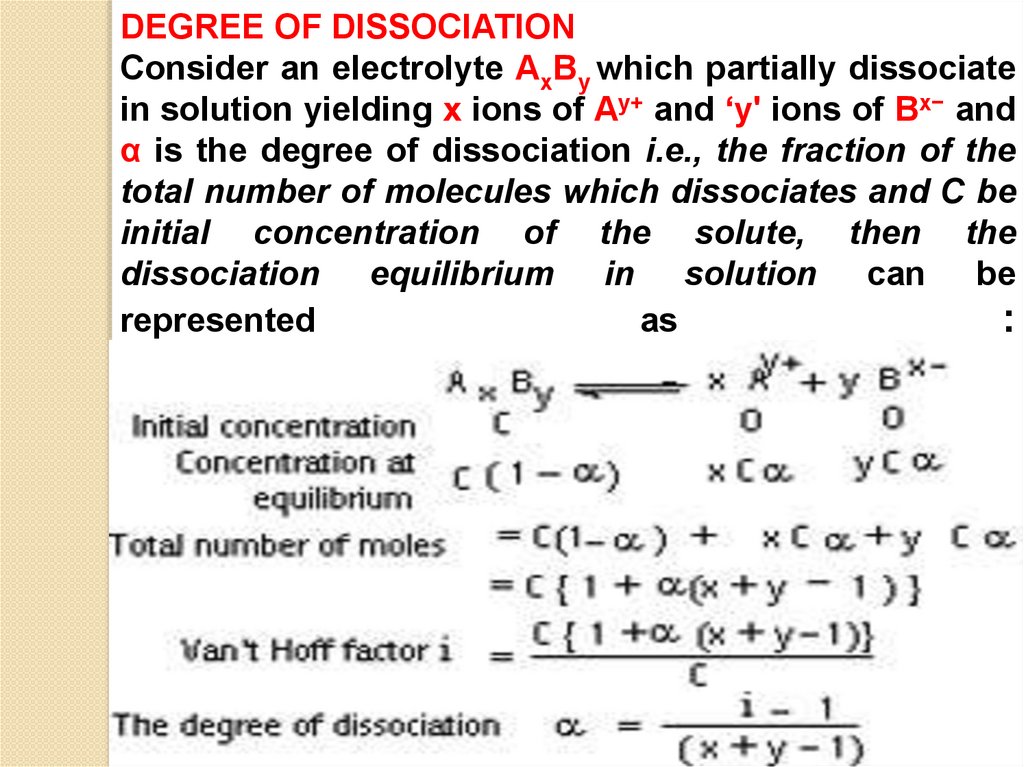
DEGREE OF DISSOCIATIONConsider an electrolyte AxBy which partially dissociate
in solution yielding x ions of Ay+ and ‘y' ions of Bx− and
α is the degree of dissociation i.e., the fraction of the
total number of molecules which dissociates and C be
initial concentration of the solute, then the
dissociation equilibrium in solution can be
represented
as
:
30.
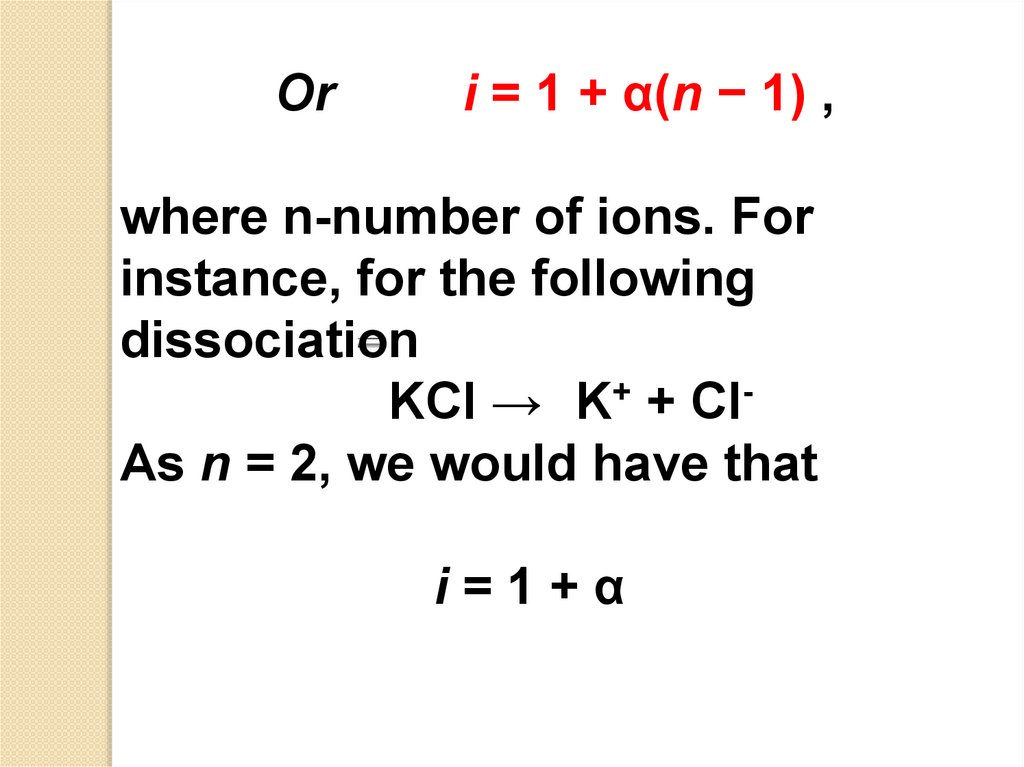
Ori = 1 + α(n − 1) ,
where n-number of ions. For
instance, for the following
dissociation
KCl → K+ + ClAs n = 2, we would have that
i=1+α
31.
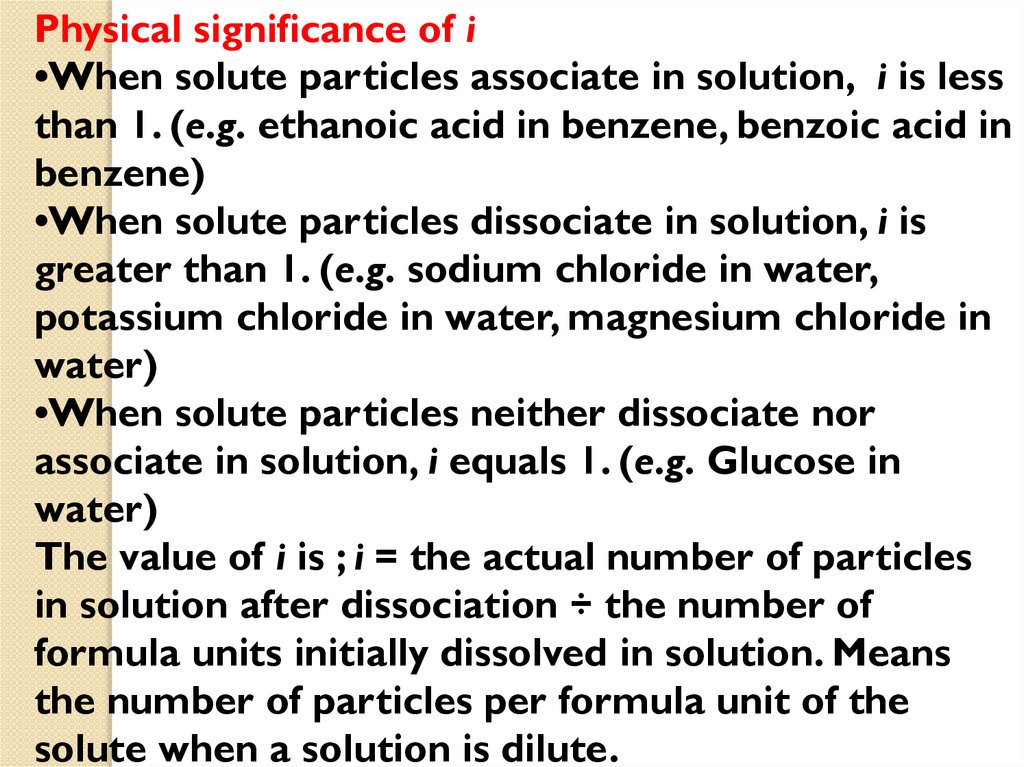
Physical significance of i•When solute particles associate in solution, i is less
than 1. (e.g. ethanoic acid in benzene, benzoic acid in
benzene)
•When solute particles dissociate in solution, i is
greater than 1. (e.g. sodium chloride in water,
potassium chloride in water, magnesium chloride in
water)
•When solute particles neither dissociate nor
associate in solution, i equals 1. (e.g. Glucose in
water)
The value of i is ; i = the actual number of particles
in solution after dissociation ÷ the number of
formula units initially dissolved in solution. Means
the number of particles per formula unit of the
solute when a solution is dilute.
32.
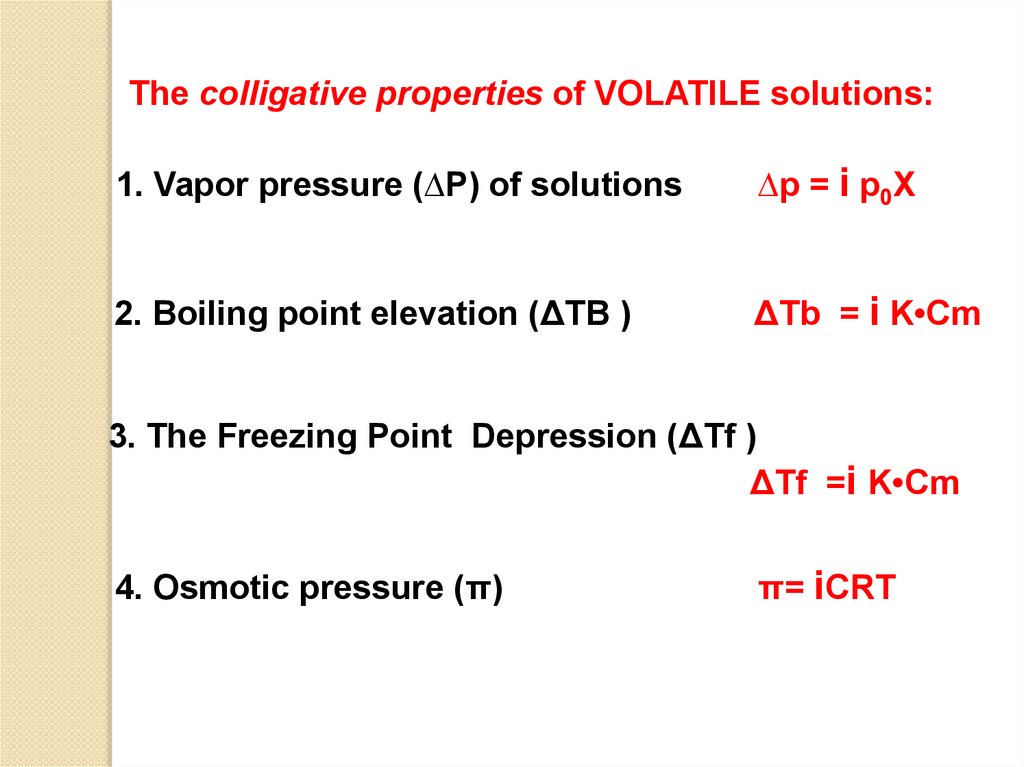
The colligative properties of VOLATILE solutions:1. Vapor pressure (∆P) of solutions
∆p = i p0Χ
2. Boiling point elevation (ΔTB )
ΔTb = i K•Cm
3. The Freezing Point Depression (ΔTf )
ΔTf =i K•Cm
4. Osmotic pressure (π)
π= iCRT












 chemistry
chemistry








