Similar presentations:
Preparation for COP
1.
Preparation for СОЧ2.
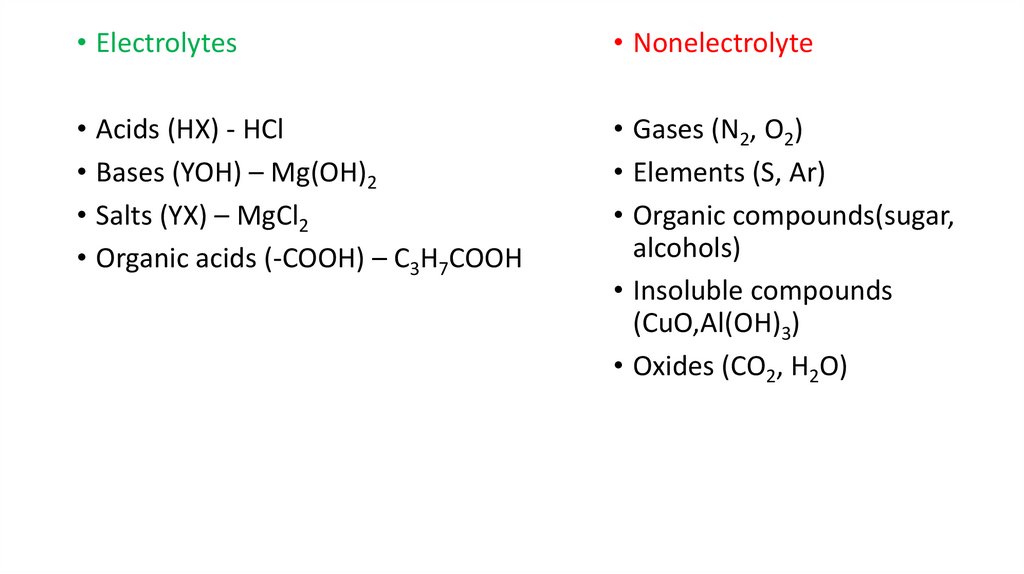
• Electrolytes• Nonelectrolyte
• Acids (HX) - HCl
• Bases (YOH) – Mg(OH)2
• Salts (YX) – MgCl2
• Organic acids (-COOH) – C3H7COOH
• Gases (N2, O2)
• Elements (S, Ar)
• Organic compounds(sugar,
alcohols)
• Insoluble compounds
(CuO,Al(OH)3)
• Oxides (CO2, H2O)
3.
Classify the compoundsPbS
C5H11COOCH3
C8H17COOH
NH3
RbOH
LiOH
H3PO4
H2CR2O7
CuSO4
LiF
F2
CO
4.
Write dissociation of the compounds• H2CrO4
• NH3
5.
Write dissociation of the compounds• Mg(OH)2
• MgOHCl
6.
Write dissociation of the compounds• Al2(HPO4)3
7.
Finish the reactions, write ionic and net ionicequations
• (NH4)2S+LiOH =
8.
Finish the reactions, write ionic and net ionicequations
• CuSO4+KOH =
9.
Finish the reactions, write ionic and net ionicequations
• RbOH+H3PO4=
10.
Chemical properties of acids• Acid + Active Metal → Salt + Hydrogen
• H2SO4 + K =
11.
Chemical properties of acids• Acid + Active Metal → Salt + Hydrogen
• H3PO4+Ba =
12.
Chemical properties of acidsAcid + Basic Oxide → Salt + Water
• H3PO4+BaO =
13.
Chemical properties of acidsAcid + Basic Oxide → Salt + Water
• H2SO4 + K2O=
14.
Chemical properties of acidsAcid + Base → Salt + Water
• H2SO4 + KOH=
15.
Chemical properties of acidsAcid + Base → Salt + Water
• H3PO4+Ba(OH)2 =
16.
Chemical properties of acidsAcid + Carbonate → Salt + Carbon dioxide + Water
• H3PO4+BaCO3 =
17.
Chemical properties of acidsAcid + Carbonate → Salt + Carbon dioxide + Water
• H2SO4 + K2CO3=
18.
Chemical properties of basesBase + Acidic Oxide → Salt + Water
P2O5+Ba(OH)2=
19.
Chemical properties of basesBase + Acidic Oxide → Salt + Water
SO3+KOH=
20.
Chemical properties of salts• Salt of 1st Metal + 2nd Metal → Salt of 2nd Metal + 1st Metal
• Mg(NO3)2+K=
Li, K, Ba, Ca, Na, Mg, Al, Mn, Zn, Cr, Fe, Co, Sn, Pb, H2, Cu, Hg, Ag, Au
21.
Chemical properties of salts• Salt of 1st Metal + 2nd Metal → Salt of 2nd Metal + 1st Metal
• Mg(NO3)2+Fe=
Li, K, Ba, Ca, Na, Mg, Al, Mn, Zn, Cr, Fe, Co, Sn, Pb, H2, Cu, Hg, Ag, Au
22.
Hydrolysis• Finish the reactions and determine the medium of the solutions
• Mg(NO3)2 + H2O =
• K2SO4 +H2O =
• Ba3(PO4)2 + H2O =
23.
What volume of hydrogen gas is produced when14 g of zinc metal reacts with 14 g of sulfuric acid
solution? Determine the mass left of excess
reagent.
24.
• Reaction goes according this eqution А+В = 2С. Initial concentrationof substance A is 0.56 mol/ l , after 20 s becomes 0,25mol/l . Calculate
average rate of the reaction
25.
• Calculate the rate of the reaction if temperature decreases from 450Ctill 250C. And the temperature coefficient is 3
26.
• Write the kinetic equations :• А) S(s) + O2 (g) = SO2 (g)
• Б) 2SO2 (g) + O2 (g) = 2SO3 (l)
27.
• How would the rate of the reaction changed if pressure decreased 4times:
• S (s) + O2 (g) = SO2 (g)
28.
• How would the rate of the reaction changed if pressure increased 2times:
• 2SО2 (g) + O2 (g) = 2SO3 (g)



























 chemistry
chemistry








