Similar presentations:
Ethics in research
1.
ETHICSIN
RESEARCH
2.
DEFINITION• ETHICS-Greek word: ethos=custom or convention, or the
spirit of community
• Moral principles that govern a person’s behaviour or the
conducting of an activity: Oxford dictionary (2014)
• The branch of philosophy that deals with morality. Ethics
is concerned with distinguishing between good and evil in
the world, between right and wrong human actions, and
between virtuous and non virtuous characteristics of
people-The American Dictionary of Cultural Literacy
(2005)
3.
WHAT ETHICS IS AND WHAT ITIS NOT
WHAT ETHICS IS
WHAT ETHICS IS NOT
• About commitment to
positive values
• About negative code of
conduct, moral prohibitions,
disciplinary rules
• A communal activity, applying
rational principles and
universal standards to social
life
• About real power relations
and responsible power
sharing
• A private matter, nor about
subjective feelings, personal
attitudes and choices
• Introspective self
examination, or judging one’s
or other’s moral state
4.
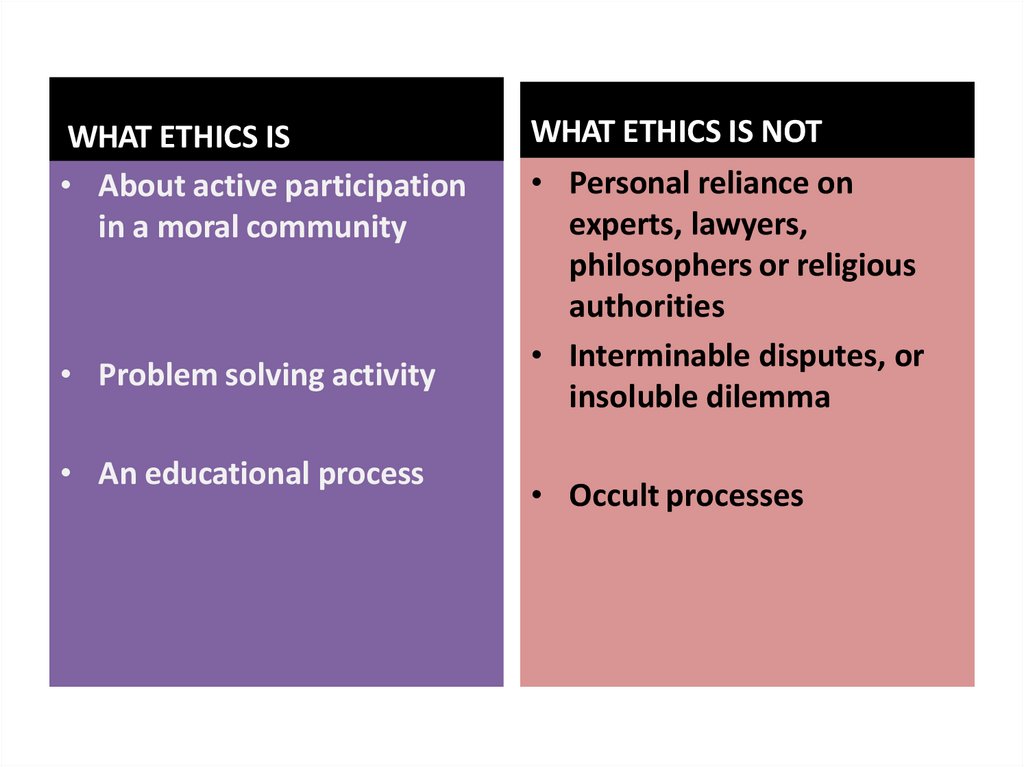
WHAT ETHICS IS• About active participation
in a moral community
• Problem solving activity
• An educational process
WHAT ETHICS IS NOT
• Personal reliance on
experts, lawyers,
philosophers or religious
authorities
• Interminable disputes, or
insoluble dilemma
• Occult processes
5.
ETHICAL THEORIES• Deontology- duty is the basis of all action
• Teleology- actions can only judged on the
basis of consequences they produce
Utilitarianism-central concern is ‘the
general welfare rather than individual’s
interest’
6.
IMPORTANCE OF ETHICS INRESEARCH
• Protects the vulnerable group and other study
participants
• Participants are safeguarded from exploitation
• Establishes risk-benefit ratio for study subjects
• Ensures fullest respect, dignity, privacy,
disclosure and fair treatment for subject
• Builds capability of subjects to accept or reject
participation in study
7.
ETHICAL PRINCIPLESThe Belmont report articulates three primary
ethical principles
Beneficence
Respect for human dignity
Justice
8.
BENEFICENCE• Imposes duty on researchers to
minimise harm and to maximise
benefits
The right to protect from harm and
discomfort
Freedom from exploitation
Benefits from research
9.
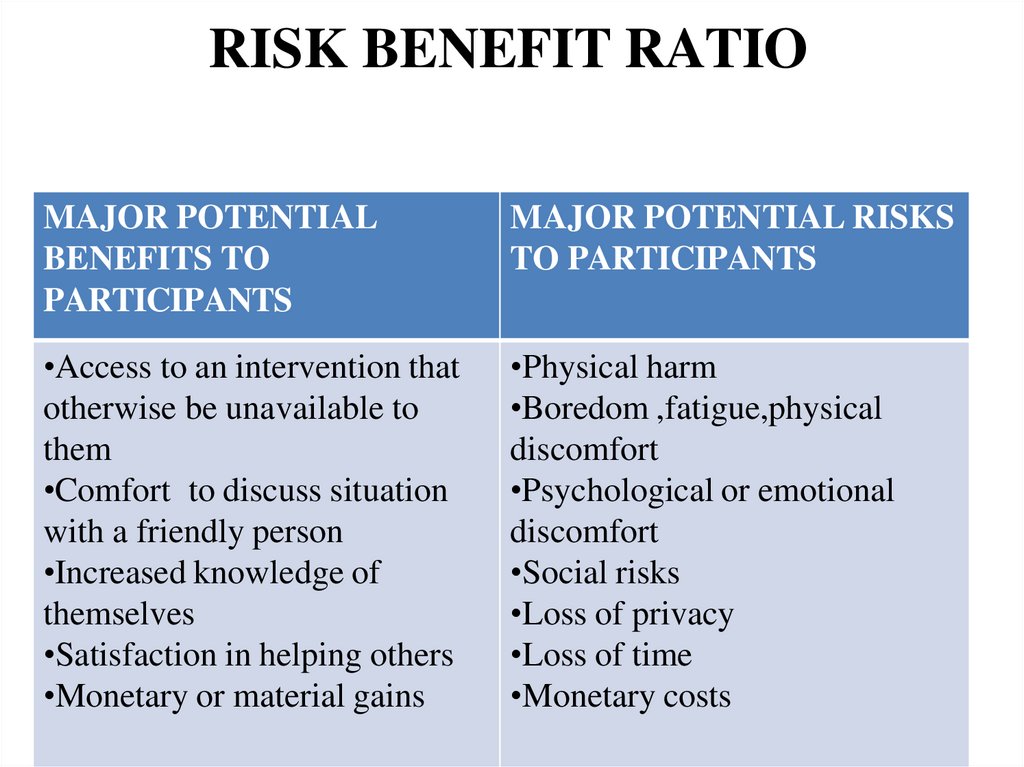
RISK BENEFIT RATIOMAJOR POTENTIAL
BENEFITS TO
PARTICIPANTS
MAJOR POTENTIAL RISKS
TO PARTICIPANTS
•Access to an intervention that
otherwise be unavailable to
them
•Comfort to discuss situation
with a friendly person
•Increased knowledge of
themselves
•Satisfaction in helping others
•Monetary or material gains
•Physical harm
•Boredom ,fatigue,physical
discomfort
•Psychological or emotional
discomfort
•Social risks
•Loss of privacy
•Loss of time
•Monetary costs
10.
THE PRINCIPLE OF RESPECT FORHUMAN DIGNITY
• The right to self determination-Humans should be
treated as autonomous agents, capable of
controlling their own activities
• The right to full disclosure-Researcher should
fully describe the nature of study, subject’s right
to refuse participation, researcher’s responsibility
and risks and benefits
11.
ISSUES RELATED TO PRINCIPLE OFRESPECT
• Inability of individuals to make well
informed judgements
• Bias
• Concealment
• Deception
12.
THE PRINCIPLE OFJUSTICE
• The right to fair
treatment
• The right to privacy
13.
INFORMED CONSENT• Participants have adequate knowledge
regarding research, have the power of
choice, enabling to decline participation
voluntarily.
• Informed assent-the process where by
minors may agree to participate in clinical
trials.
14.
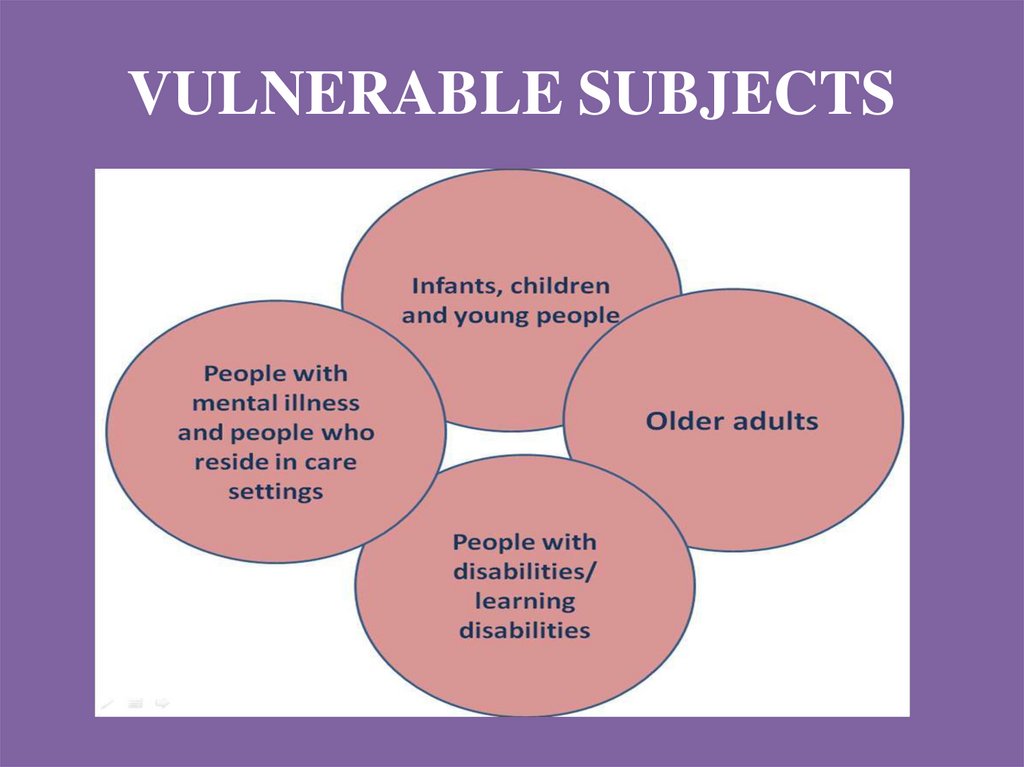
VULNERABLE SUBJECTS15.
THERAPEUTICMISCONCEPTION
• Research subject misinterpret and enrol in
the study thinking it to be routine medical
care
• Misinterpret the information and believes
that research directly benefits him
16.
POST TRIAL ACCESS• Holds special importance for clinical
research
• Pharmaceutical companies from developed
countries collect data from developing
countries
• Most of these drugs would never be used by
the communities from where the
experimental data is collected
17.
CODE OF ETHICS FORDIFFERENT DISCIPLINES
18.
19781992
1995
• Important Code of ethics adopted by National
Commission for the Protection of Human
Subjects of Biomedical and Behavioural
Research (U. S)
• Guidelines for psychologists published by the
American Psychological Association in Ethical
principles of Psychologists and Code of Conduct
• The American Nurses’Association put forth a
document entitled Ethical guidelines in the
Conduct, Dissemination and Implementation of
Nursing Research
19.
ETHICAL CONCERNS INQUALITATIVE RESEARCH
• Distress
• Misinterpretation
• Identification
• Inconvenience
20.
• ETHICAL CONCERNSIN QUANTITATIVE
RESEARCH
Related to the stage of
research
• Formulating the research
questions
• Designing the study
• Collecting data
• Analysis
• Reporting
21.
ETHICAL CONCERNS INMIXED METHOD
RESEARCH
• Identify and describe issues
related to the protection of
human subjects
• Understand the ethical issues
associated with quantitative
and qualitative research
• Be prepared to educate IRB
reviewers about mixed
method research
22.
STUDENTS’ ROLE IN ETHICSIN RESEARCH
• Ethical clearance should be
get done
• Need to get approval from
guides and co-guides
• Unethical to publish
including guide as coauthor
23.
INTERNET ETHICS• Development of internet over
years led to use of internet
based research.
• Numerous approaches
include web page content
analysis, online focus groups,
online interviews, analysis of
e-conversations
24.
CONCLUSION• If research is based on
a robust design and in
a safe and ethical
manner, it can be of
benefit to all
• Professional codes,
laws, regulations, and
ethics committees can
provide guidance but
ultimate determinant
rests with researcher’s
value system and moral
code






















 education
education








