Similar presentations:
The Most Complete Interpretation of Anode Materials Standards for Lithium-ion Batteries
1.
The Most Complete Interpretation of AnodeMaterials Standards for Lithium-ion Batteries
With many advantages, such as high energy density, long cycle life, low self-discharge,
no memory effect and environmental friendliness, lithium-ion batteries (LIBs) have been
widely used in consumer electronics, such as smartphones, smart bracelets, digital cameras
and laptops, with the strongest consumer demand. At the same time, it is promoted in the
markets of pure electric, hybrid electric and extended-range electric vehicles, with the fastest
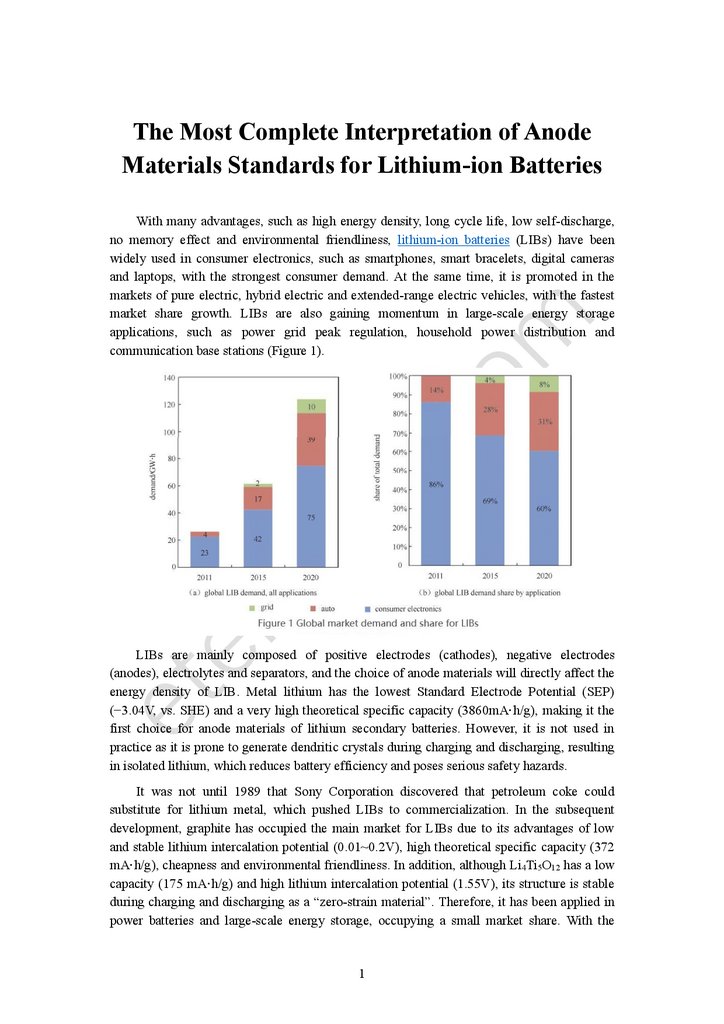
market share growth. LIBs are also gaining momentum in large-scale energy storage
applications, such as power grid peak regulation, household power distribution and
communication base stations (Figure 1).
LIBs are mainly composed of positive electrodes (cathodes), negative electrodes
(anodes), electrolytes and separators, and the choice of anode materials will directly affect the
energy density of LIB. Metal lithium has the lowest Standard Electrode Potential (SEP)
(−3.04V, vs. SHE) and a very high theoretical specific capacity (3860mA·h/g), making it the
first choice for anode materials of lithium secondary batteries. However, it is not used in
practice as it is prone to generate dendritic crystals during charging and discharging, resulting
in isolated lithium, which reduces battery efficiency and poses serious safety hazards.
It was not until 1989 that Sony Corporation discovered that petroleum coke could
substitute for lithium metal, which pushed LIBs to commercialization. In the subsequent
development, graphite has occupied the main market for LIBs due to its advantages of low
and stable lithium intercalation potential (0.01~0.2V), high theoretical specific capacity (372
mA·h/g), cheapness and environmental friendliness. In addition, although Li4Ti5O12 has a low
capacity (175 mA·h/g) and high lithium intercalation potential (1.55V), its structure is stable
during charging and discharging as a “zero-strain material”. Therefore, it has been applied in
power batteries and large-scale energy storage, occupying a small market share. With the
1
2.
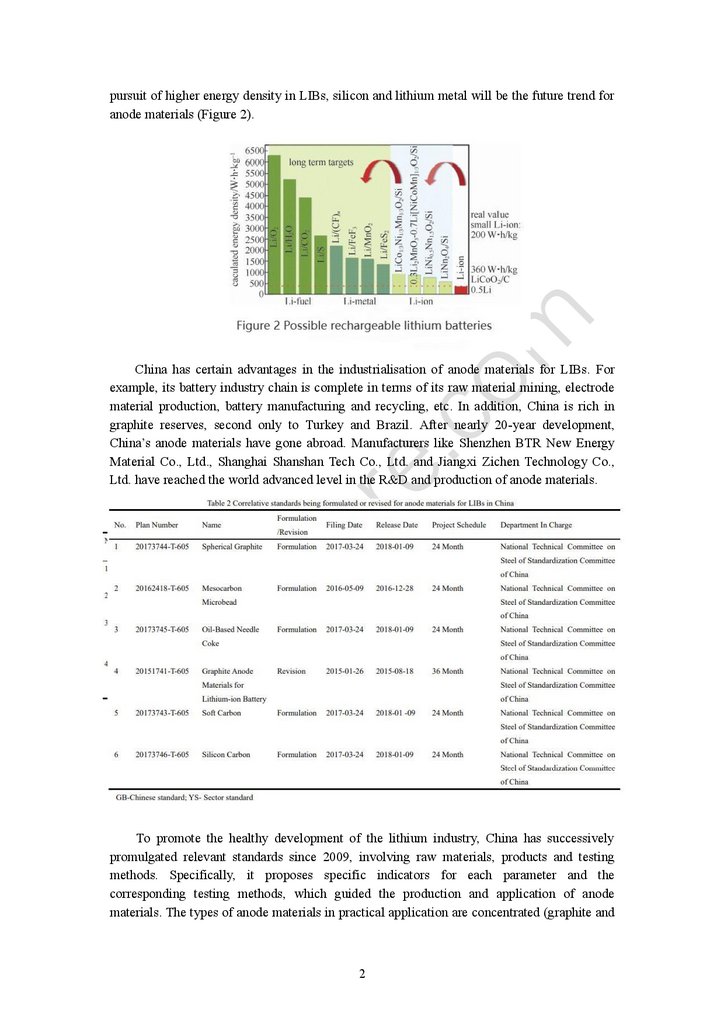
pursuit of higher energy density in LIBs, silicon and lithium metal will be the future trend foranode materials (Figure 2).
China has certain advantages in the industrialisation of anode materials for LIBs. For
example, its battery industry chain is complete in terms of its raw material mining, electrode
material production, battery manufacturing and recycling, etc. In addition, China is rich in
graphite reserves, second only to Turkey and Brazil. After nearly 20-year development,
China’s anode materials have gone abroad. Manufacturers like Shenzhen BTR New Energy
Material Co., Ltd., Shanghai Shanshan Tech Co., Ltd. and Jiangxi Zichen Technology Co.,
Ltd. have reached the world advanced level in the R&D and production of anode materials.
To promote the healthy development of the lithium industry, China has successively
promulgated relevant standards since 2009, involving raw materials, products and testing
methods. Specifically, it proposes specific indicators for each parameter and the
corresponding testing methods, which guided the production and application of anode
materials. The types of anode materials in practical application are concentrated (graphite and
2
3.
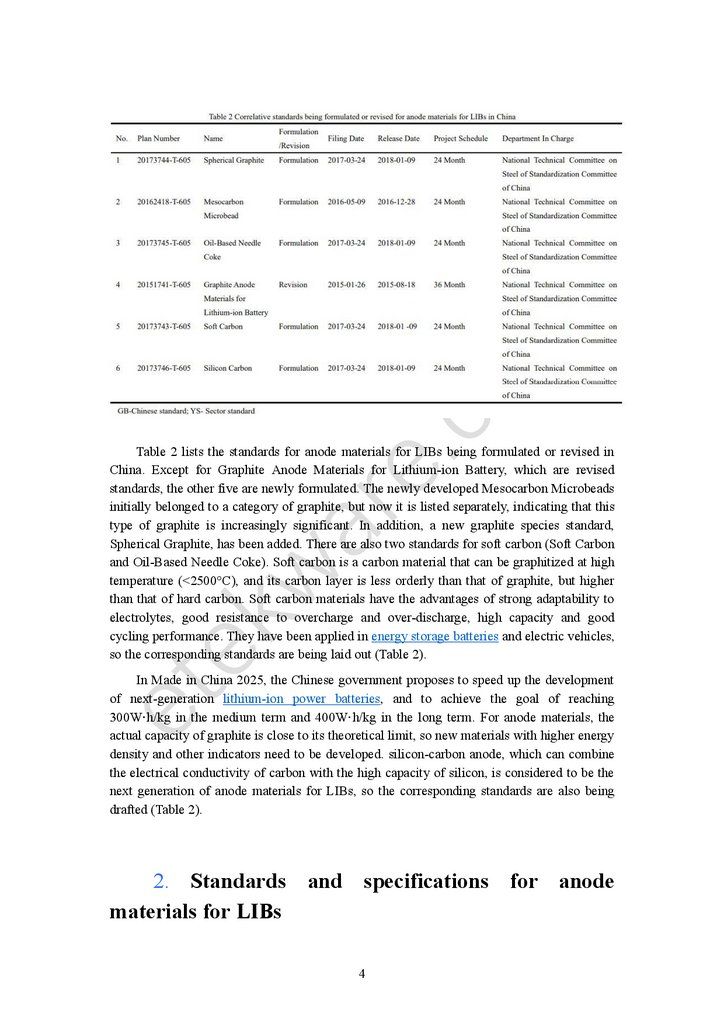
Li4Ti5O12), related to four standards (Table 1). Now, there are six standards underdevelopment or revision (Table 2), indicating that the variety of anode materials has increased
and new standards are needed to regulate their development. This article will focus on the
main points of the four promulgated standards.
1. Correlative standards for anode materials for
LIBs in China
Table 1 lists the relevant standards for anode materials for LIBs released in China in the
last decade or so, including three national standards and one industry standard. In terms of
categories, there are three anode products and one test method involved. Graphite was the
first anode material to be commercialized, so GB/T24533-2009 Graphite Anode Materials for
Lithium-ion Battery was the first anode standard. Subsequently, a small amount of lithium
titanate entered the market, with the corresponding industry standard YS/T825-2012 Lithium
Titanium and national standard GB/T30836-2014 Lithium Titanium Oxide and Its Carbon
Composite Anode Materials for Lithium-ion Battery launched successively.
Graphite Anode Materials for Lithium-ion Battery divides graphite into natural graphite,
mesocarbon microbead artificial graphite, needle coke artificial graphite, petroleum coke
artificial graphite and composite graphite. Specifically, each category is divided into different
grades according to its electrochemical performance (the first charge-discharge efficiency and
the first coulombic efficiency), and each grade is divided into different varieties according to
the average particle size (D50) of its material. The standard sets out requirements for various
physicochemical properties of different varieties of graphite. Due to space limitations, the
following part only divides graphite into natural graphite, mesocarbon microbead artificial
graphite, needle coke artificial graphite, petroleum coke artificial graphite and composite
graphite. Each category of indicators combines all the parameters of different grades and
varieties of the graphite in one category.
3
4.
Table 2 lists the standards for anode materials for LIBs being formulated or revised inChina. Except for Graphite Anode Materials for Lithium-ion Battery, which are revised
standards, the other five are newly formulated. The newly developed Mesocarbon Microbeads
initially belonged to a category of graphite, but now it is listed separately, indicating that this
type of graphite is increasingly significant. In addition, a new graphite species standard,
Spherical Graphite, has been added. There are also two standards for soft carbon (Soft Carbon
and Oil-Based Needle Coke). Soft carbon is a carbon material that can be graphitized at high
temperature (<2500°C), and its carbon layer is less orderly than that of graphite, but higher
than that of hard carbon. Soft carbon materials have the advantages of strong adaptability to
electrolytes, good resistance to overcharge and over-discharge, high capacity and good
cycling performance. They have been applied in energy storage batteries and electric vehicles,
so the corresponding standards are being laid out (Table 2).
In Made in China 2025, the Chinese government proposes to speed up the development
of next-generation lithium-ion power batteries, and to achieve the goal of reaching
300W·h/kg in the medium term and 400W·h/kg in the long term. For anode materials, the
actual capacity of graphite is close to its theoretical limit, so new materials with higher energy
density and other indicators need to be developed. silicon-carbon anode, which can combine
the electrical conductivity of carbon with the high capacity of silicon, is considered to be the
next generation of anode materials for LIBs, so the corresponding standards are also being
drafted (Table 2).
2. Standards and specifications for anode
materials for LIBs
4
5.
2.1 Requirements for anode materials for LIBsAnode materials, the core component of LIBs, are needed when the following conditions
are met:
①Low and stable lithium intercalation potential to ensure high output voltage;
②High specific capacity to allow more Li-ions to be reversibly deintercalated;
③Stable structure during charging and discharging, with a long cycle life;
④High electronic conductivity, ionic conductivity and low charge transfer resistance to
ensure small voltage polarization and good rate capability;
⑤Ability to form a solid electrolyte membrane (SEI) with the electrolyte to ensure high
coulombic efficiency;
⑥Simple preparation process, easy industrialisation and inexpensiveness;
⑦Environmentally friendliness, with no serious pollution to the environment during the
production and usage of materials;
⑧Abundant resources, etc.
For more than 30 years, although new anode materials for LIBs have been reported, few
have been commercially available, because few materials can take the above conditions into
account. For example, although metal oxides, sulphides and nitrides have high specific
capacity, their high potential, severe polarisation, considerable volume changes, difficulty in
forming stable SEI and high cost during lithium intercalation prevent them from being applied.
Graphite is widely used because it balances the above conditions. In addition, although
Li4Ti5O12 has low capacity and high lithium intercalation potential, its structure is stable
during charge and discharge, allowing high-rate charge and discharge, so it also has been
applied in power batteries and large-scale energy storage.
The production of anode materials is one part of the entire battery manufacturing process,
and the formulation of their standards will help battery enterprises to judge the material
quality. In addition, as materials are inevitably affected by people, machines, materials,
environment, test conditions, etc. during production and transport. Only by standardizing their
physical and chemical properties can their reliability be ensured.
Generally, the key technical indicators of anode materials include crystal structure,
particle size distribution, tapped density, specific surface area, pH, water content, major
element content, impurity element content, first discharge specific capacity and first chargedischarge efficiency, etc. They will be explained below.
5
6.
2.2 Crystal structure of anode materialsGraphite mainly has two crystal structures, a hexagonal phase (a=b=0.2461nm, c=0.6708
nm, α=β=90°, γ=120°, P63/mmc space group) and a rhombohedral phase (a=b=c, α=β=γ≠90°,
R3m space group) (Table 3). In graphite crystals, these two structures co-exist, but their ratio
varies in different graphite materials, which can be determined by the X-ray diffraction test.
For carbon materials, the degree of ordering of the crystal structure and the difficulty of
graphitization can be described by the degree of graphitization (G). The larger the G, the
easier the graphitization of the carbon material, and the higher the degree of ordering of the
crystal structure. Specifically, d002 is the interplanar spacing of the (002) peak in the XRD
pattern of carbon materials, 0.3440 is the interplanar spacing of completely ungraphitised
carbon, and 0.3354 is the interplanar spacing of ideal graphite (all units are nm). The above
formula shows that the smaller the d002 of the carbon material, the higher the degree of
graphitization, the fewer the corresponding lattice defects, the smaller the electron migration
resistance, and the dynamic performance of the battery will be improved. Therefore, the d002
values for each type of graphite are clearly defined in GB/T24533-2009 Graphite Anode
Materials for Lithium-ion Battery (Table 3).
Li4Ti5O12 is a cubic spinel, belonging to the Fd-3m space group, with three-dimensional
Li-ion migration channels (Figure 4). Compared with the structure of its lithium intercalation
product (Li7Ti5O12), the unit cell parameters of Li4Ti5O12, known as a “zero-strain material”,
have little difference (0.836nm→0.837nm), which results in excellent cycling stability.
6
7.
Generally, Li4Ti5O12 is prepared by high-temperature sintering with TiO2 and Li2CO3 asraw materials, so there may be a small amount of TiO2 residue in the product, which affects
the electrochemical performance of the material. For this reason, GB/T30836-2014 Lithium
Titanium Oxide and Its Carbon Composite Anode Materials for Lithium-ion Battery gives the
upper limit of TiO2 residue in Li4Ti5O12 products and the detection method. The specific
process is as follows. Firstly, the diffraction pattern of the sample measured by XRD should
comply with JCPDS (49-0207). Secondly, the intensity of the (111) crystalline diffraction
peak, the anatase TiO2 (101) crystalline diffraction peak and the rutile TiO2 (110) crystalline
diffraction peak of Li4Ti5O12 are read out from the spectrum. Finally, after the intensity of the
anatase TiO2 peak I101/I111 and the rutile TiO2 peak I110/I111 TiO2 are calculated, judgement can
be made against the requirements of the standard (Table 3).
2.3 Particle size distribution of anode materials
The particle size distribution of the anode material directly affects the pulping technics
and volumetric energy density of the battery. In the case of the same volume filling fraction,
the larger the particle size of the material, the wider the particle size distribution, and the
smaller the viscosity of the slurry (Figure 5), which helps to increase the solid content and
reduce the difficulty of coating. In addition, with a wider particle size distribution, the small
particles in the system can fill the voids of the large particles, which helps to increase the
compacted density of the pole piece and to improve the volumetric energy density of the
battery.
7
8.
The particle size and size distribution of materials can be measured by laser diffractionparticle size analysers and nanoparticle analysers. The laser diffraction particle size analyser
works is used to measure the particle system at the micron level based on static light
scattering theory, i.e., particles of different sizes scatter light at different angles with different
intensities. The nanoparticle analyser works based on dynamic light scattering theory, i.e., the
more severe Brownian motion of nanoparticles affects not only the intensity of scattered light,
but also its frequency, and thus the particle size distribution of the nanoparticles is determined.
The characteristic parameters of the material size distribution are D50, D10, D90 and Dmax.
D50 is the particle size corresponding to 50% of the cumulative amount in the particle size
cumulative distribution curve, which can be regarded as the average particle size of the
material. In addition, the width of the material size distribution can be expressed by K90 (K90=
(D90 - D10)/D50). The larger the K90, the wider the distribution.
The particle size of the anode material is mainly determined by its preparation method.
For example, the synthesis method of mesocarbon microspheres (MCMBs) is the thermal
decomposition and thermal polycondensation of liquid-phase hydrocarbons under high
temperature and high pressure. By controlling the type of raw materials, reaction time,
temperature, pressure, etc., the particle size of MCMBs can be regulated. The requirements
for particle size parameters in the graphite standard are D50 (~20μm), Dmax (≤70μm) and D10
(~10μm), while the D50 required in the lithium titanate standard is considerably smaller than
that of graphite (≤10μm, Table 4).
2.4 Density of anode materials
Generally, powder materials are porous, some with the outer surface of the particle,
known as open pores or semi-open pores (connected at one end), and some not connected to
the outer surface at all, known as closed pores. When calculating the density of a material, it
can be divided into true density, effective density and apparent density according to whether
the pore volumes are included, while apparent density is divided into compacted density and
8
9.
tapped density.The true density is the theoretical density of the powder material, so the volume used in
the calculation is the particle volume excluding open and closed pores. The effective density
is the density that the powder material can be effectively used, so the volume used is the
particle volume including the closed pores. The effective volume is measured by the
following steps. First, place the powder material in a measuring vessel. Then, add a liquid
medium and allow it to fully infiltrate the open pores of the particles. Last, subtract the
volume of the liquid medium from the measured volume to obtain the effective volume.
In practice, manufacturers are more concerned about the apparent density of the material,
including tapped density and compacted density. The principle of the compacted density test
is as follows. First, a certain amount of powder is filled in the compacted density tester, which
is continuously vibrated and rotated by the vibration device until the volume of the sample no
longer decreases. Then, the compacted density is obtained by dividing the mass of the sample
by the compacted volume.
The test principle of compacted density is as follows. During the extrusion process of
external force, as the powder moves and deforms, large voids are filled, and the contact area
between particles increases, thereby forming a compacted embryo with a certain density and
strength. The volume of the compacted embryo is the compacted volume. Generally, true
density>effective density>compacted density>tapped density.
The density of the anode material directly affects the volumetric energy density of the
battery. For the same material, the higher the compacted density, the higher the volumetric
energy density, so the lower limits for each density are specified in the standard (Table 5). The
true densities of different graphite materials are in the range of 2.20~2.26g/cm 3, because they
are essentially carbon materials, with different microstructures. In addition, due to the low
initial conductivity of Li4Ti5O12, carbon coating is required to improve the rate capability of
the battery, but meanwhile, the corresponding tapped density decreases (Table 5).
9
10.
2.5 Specific surface area of anode materialsSurface area is divided into the external and internal surface area, and the specific
surface area of a material is the total area per unit mass. The ideal non-porous material has
only an external surface area, with a small specific surface area, while the porous and multiporous materials have a large internal surface area and a high specific surface area. The pore
size of powder materials is divided into three categories: micropores (<2nm), mesopores (250nm) and macropores (>50nm). In addition, the specific surface area of a material is closely
related to its particle size. The smaller the particle size, the larger the specific surface area.
Generally, the pore size and specific surface area of the material are determined by
nitrogen adsorption and desorption experiments. The basic principle is that when the gas
molecules collide with the powder material, they will stay on the surface of the material for a
period, which is called adsorption. The amount of adsorption at constant temperature depends
on the properties of the powder and gas and the pressure when the adsorption occurs. The
specific surface area, pore size distribution and pore volume of the material can be calculated
from the adsorption amount. In addition, as the adsorption capacity of the powder to the gas
will increase as the temperature decreases, the adsorption experiment is generally carried out
at a low temperature (using liquid nitrogen) to improve the adsorption capacity of the material
for gases.
The specific surface area of the anode material has a great impact on the kinetic
performance of the battery and the formation of the solid electrolyte membrane (SEI). For
example, nanomaterials have been extensively investigated for their high specific surface area,
which can shorten the transport path of Li-ions, reduce surface current density and improve
the kinetic performance of the cell. Often, however, these materials are not used in practice,
because the large specific surface area exacerbates the breakdown of the electrolyte during the
first cycle, resulting in a low first-time coulombic efficiency. Therefore, the anode material
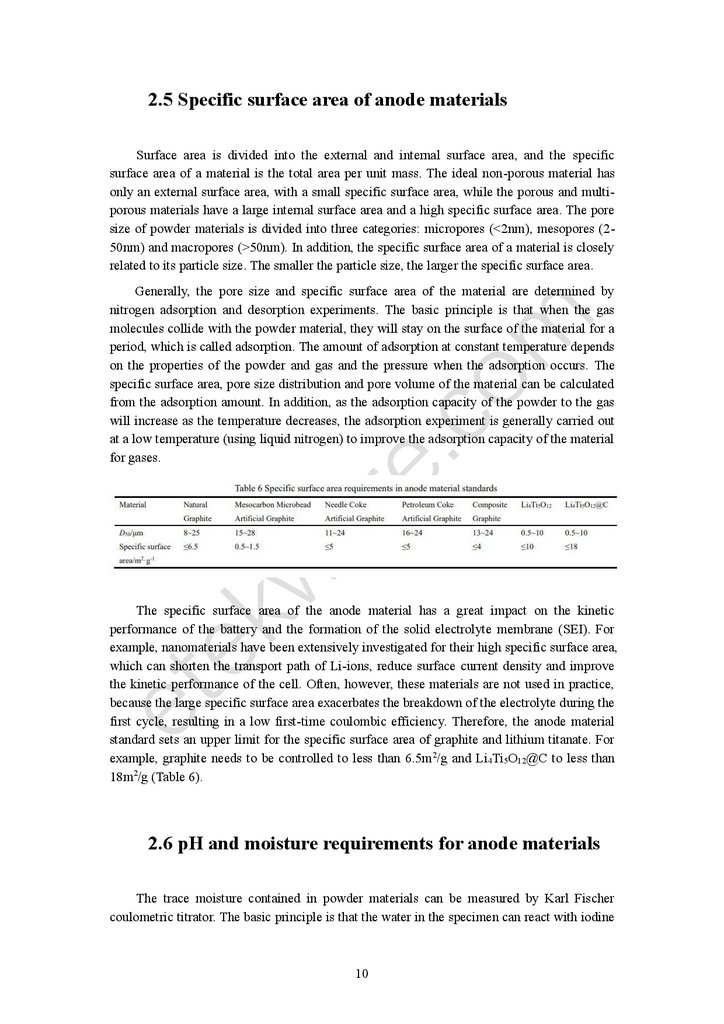
standard sets an upper limit for the specific surface area of graphite and lithium titanate. For
example, graphite needs to be controlled to less than 6.5m2/g and Li4Ti5O12@C to less than
18m2/g (Table 6).
2.6 pH and moisture requirements for anode materials
The trace moisture contained in powder materials can be measured by Karl Fischer
coulometric titrator. The basic principle is that the water in the specimen can react with iodine
10
11.
and sulphur dioxide in the presence of an organic base and methanol(H2O+I2+SO2+CH3OH+3RN→[RHN]SO4CH3+2[RHN]I), and iodine is produced by
electrochemical oxidation of the electrolytic cell (2I−—→I2+2e−). The amount of iodine
produced is proportional to the amount of electricity passing through the electrolytic cell, so
the water content can be calculated by recording the power consumed by the electrolytic cell.
The pH and moisture of the anode material have effects on the stability of the material
and the pulping technics. The pH of graphite is around neutral (4~9), while that of Li4Ti5O12
is alkaline (9.5~11.5) with a certain residual alkalinity (Table 7). It is because in the
preparation of Li4Ti5O12, to ensure that the reaction proceeds adequately, the lithium source is
excessive, which mainly exist in the form of Li2CO3 or LiOH, making the final product
alkaline. When the amount of residual alkali is too high, the stability of the material becomes
poor, so it is easy to react with water and carbon dioxide in the air, which directly affects the
electrochemical performance of the material. In addition, since the graphite-based anode
slurry is an aqueous system, its requirement for moisture (≤0.2%) is not as harsh as that of the
cathode material (the slurry is an oily system, ≤0.05%), which is of significance in reducing
the production cost of the battery and simplifying the process.
2.7 Major element content of anode material
Although the graphite anode has a high capacity and low and stable lithium intercalation
potential, it is very sensitive to the composition of the electrolyte, easy to peel off and has
poor resistance to overcharging. As a result, the commercially used graphite is modified
graphite. The modification methods include surface oxidation, surface coating, etc., which
leaves some impurities in the graphite. Graphite is composed of fixed carbon, ash and volatile
component. Fixed carbon is the electrochemically active component. The standard requires
that the content of fixed carbon needs to be more than 99.5% (Table 8), which can be
determined by indirect determination of carbon.
For Li4Ti5O12, the theoretical content of lithium is 6%, with an allowed deviation of
5%~7% in the actual product (Table 8). The content of elements can be measured by electroncoupled plasma atomic emission spectrometry, and the basic principle is as follows. First, the
working gas (Ar) generates plasma in the presence of high-frequency current. Then, the
sample interacts with the high-temperature plasma to emit photons. The wavelength of
photons is related to the elemental species, which can be determined from the excitation
wavelength. In addition, as Li4Ti5O12 has low electrical conductivity, carbon coating strategy
is adopted to enhance the reaction kinetics of the battery. The coated carbon layer should not
be too thick, as it will not only affect the migration rate of lithium ions, but also reduce the
tapped density of the material. Therefore, the carbon content is limited to less than 10% in the
standard (Table 8).
11
12.
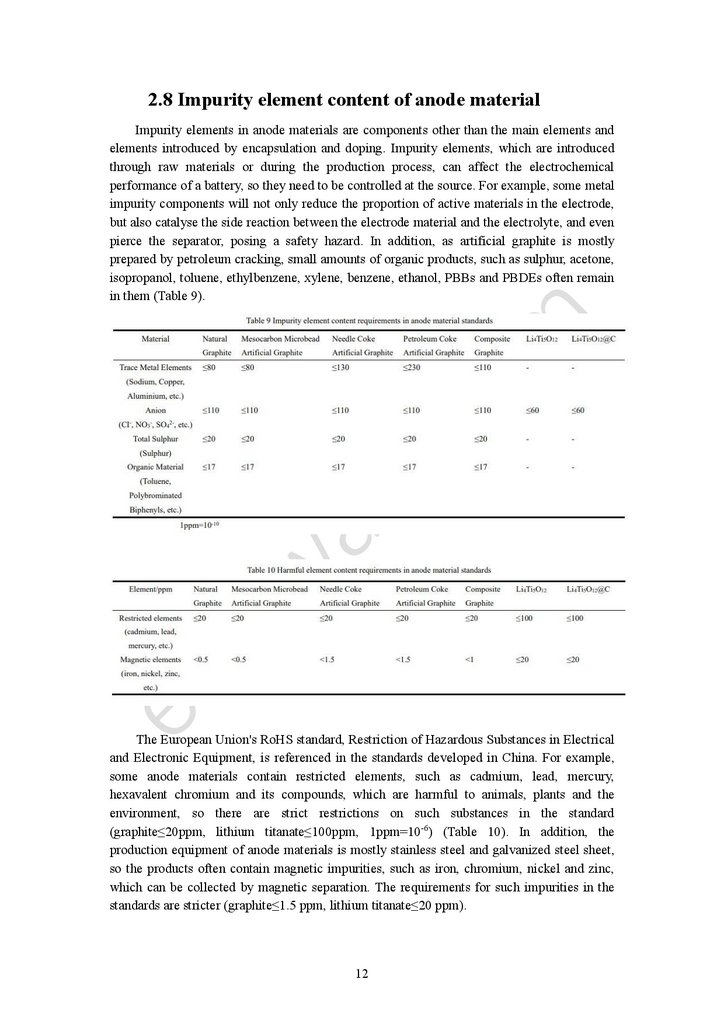
2.8 Impurity element content of anode materialImpurity elements in anode materials are components other than the main elements and
elements introduced by encapsulation and doping. Impurity elements, which are introduced
through raw materials or during the production process, can affect the electrochemical
performance of a battery, so they need to be controlled at the source. For example, some metal
impurity components will not only reduce the proportion of active materials in the electrode,
but also catalyse the side reaction between the electrode material and the electrolyte, and even
pierce the separator, posing a safety hazard. In addition, as artificial graphite is mostly
prepared by petroleum cracking, small amounts of organic products, such as sulphur, acetone,
isopropanol, toluene, ethylbenzene, xylene, benzene, ethanol, PBBs and PBDEs often remain
in them (Table 9).
The European Union's RoHS standard, Restriction of Hazardous Substances in Electrical
and Electronic Equipment, is referenced in the standards developed in China. For example,
some anode materials contain restricted elements, such as cadmium, lead, mercury,
hexavalent chromium and its compounds, which are harmful to animals, plants and the
environment, so there are strict restrictions on such substances in the standard
(graphite≤20ppm, lithium titanate≤100ppm, 1ppm=10-6) (Table 10). In addition, the
production equipment of anode materials is mostly stainless steel and galvanized steel sheet,
so the products often contain magnetic impurities, such as iron, chromium, nickel and zinc,
which can be collected by magnetic separation. The requirements for such impurities in the
standards are stricter (graphite≤1.5 ppm, lithium titanate≤20 ppm).
12
13.
2.9 First reversible specific capacity and first efficiencyof anode materials
The first reversible specific capacity of the anode material is the first-cycle delithiation
capacity, and the first efficiency is the ratio of the first-cycle delithiation capacity to the
lithium intercalation capacity, which can reflect the electrochemical performance of the
electrode material. During the first-cycle of lithium intercalation, the graphite anode will
decompose the electrolyte to form an SEI film, which allows the passage of Li-ions and
hinders the passage of electrons, which prevents further depletion of the electrolyte, thus
broadening the electrochemical window of the electrolyte.
However, the generation of an SEI film also results in a large irreversible capacity,
reducing the first coulombic efficiency. Especially, for full cells, a low first coulombic
efficiency means the loss of a limited source of lithium. In contrast, Li 4Ti5O12 has a higher
lithium intercalation potential (~1.55V) and will not generate SEI film in the first circle, so its
first efficiency is higher than that of graphite (≥90%, Table 11). The first efficiency of highquality Li4Ti5O12 can reach more than 98%. In addition, the first-cycle reversible specific
capacity of the cell can reflect the stable capacity of the material in subsequent cycles, which
is of practical importance.
3. Recommendations for future standard-setting
work
With the basic principle of practicality, the formulation of standards helps to serve
enterprises and meet market demands. However, the current LIB electrode material products
are changing rapidly, bringing challenges to the development of standards. Taking the
currently implemented Graphite Anode Materials for Lithium-ion Battery as an example. The
standard involves five categories: natural graphite, mesophase carbon microsphere artificial
graphite, needle coke artificial graphite, petroleum coke artificial graphite and composite
graphite. Each category is divided into different varieties according to its electrochemical
properties and average particle size. However, these criteria are not well applied from the
customer's point of view.
In addition, the standard contains too much content and is not so targeted. It is suggested
that separate standards be established for natural graphite, intermediate phase carbon
microsphere artificial graphite, needle coke artificial graphite, petroleum coke artificial
13
14.
graphite and composite graphite. In addition, neither the rate performance nor the cycle life ofthe anode materials is clearly defined in the standard. As these two indicators are also the key
parameters to measure whether the electrode material can be practically applied, it is
suggested that these two indicators be added to the subsequent standards.
Raw materials and testing methods are important factors for battery consistency. For LIB
cathode materials, there are independent standards for raw materials (e.g. lithium carbonate,
lithium hydroxide and cobaltosic oxide) and test methods (e.g. lithium cobaltate
electrochemical performance tests – first discharge specific capacity and first chargedischarge efficiency test methods). However, few such standards have been addressed for the
anode side of LIBs. As the performance of different anode materials varies considerably, it is
necessary to be specific in the testing methods. Therefore, it is recommended to formulate
independent standards for different raw materials of anode materials and different anode
material testing methods in the future.
For silicon anodes, there are two technical routes, nano-silicon carbon and silicon oxide,
whose basic performance differs considerably. The first coulombic efficiency and specific
capacity of the nano-silicon carbon anode are high, but its volume expansion is large and the
cycle life is low, while the volume expansion of silicon oxide is small and the cycle life is
better, but the first efficiency is low. As the exact route to be developed also depends on the
market and customer demand for the product, it is suggested that the formulation of the
standard for silicon anode should be divided into two different systems: nano-silicon carbon
and silicon oxide, so that the parameters in the standard are more pertinent and practical.
In addition, hard carbon is a conventional anode material for LIBs. It is used in a narrow
range, mainly by incorporating graphite anode to improve the rate performance of anode
materials. However, in the future, the market share of hard carbon may gradually increase as
the applications of LIBs diversify. Therefore, it can be standardized at the right time. Also,
lithium-sulphur and lithium-air batteries are new battery systems with high energy density, so
metal lithium is also the future direction for the development of anode materials. However, as
the development of lithium metal batteries is still in its infancy and will not be widely used in
the short term, it is still too early to formulate standards for lithium metal anodes.
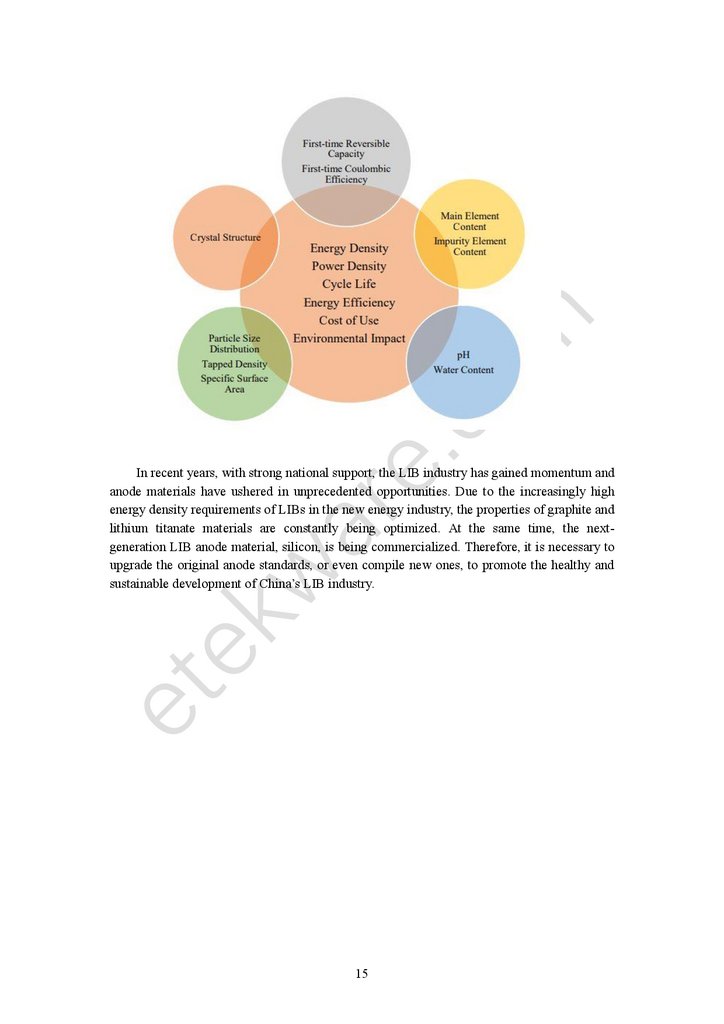
Conclusion
To sum up, the standard of anode material is mainly based on five aspects: crystal
structure, particle size distribution, tapped density and specific surface area, pH and water
content, main and impurity element contents, first reversible specific capacity and first
charge-discharge efficiency, to achieve high energy density, high power density, long cycle
life, high energy efficiency, low cost of use, and environmental friendliness in batteries
(Figure 6). These standards regulate the parameters of anode materials for LIBs, which can be
used to guide their production and application.
14
15.
In recent years, with strong national support, the LIB industry has gained momentum andanode materials have ushered in unprecedented opportunities. Due to the increasingly high
energy density requirements of LIBs in the new energy industry, the properties of graphite and
lithium titanate materials are constantly being optimized. At the same time, the nextgeneration LIB anode material, silicon, is being commercialized. Therefore, it is necessary to
upgrade the original anode standards, or even compile new ones, to promote the healthy and
sustainable development of China’s LIB industry.
15