Similar presentations:
Биосенсоры. Иммобилизация фермента на поверхности электрода
1.
Biosensors2.
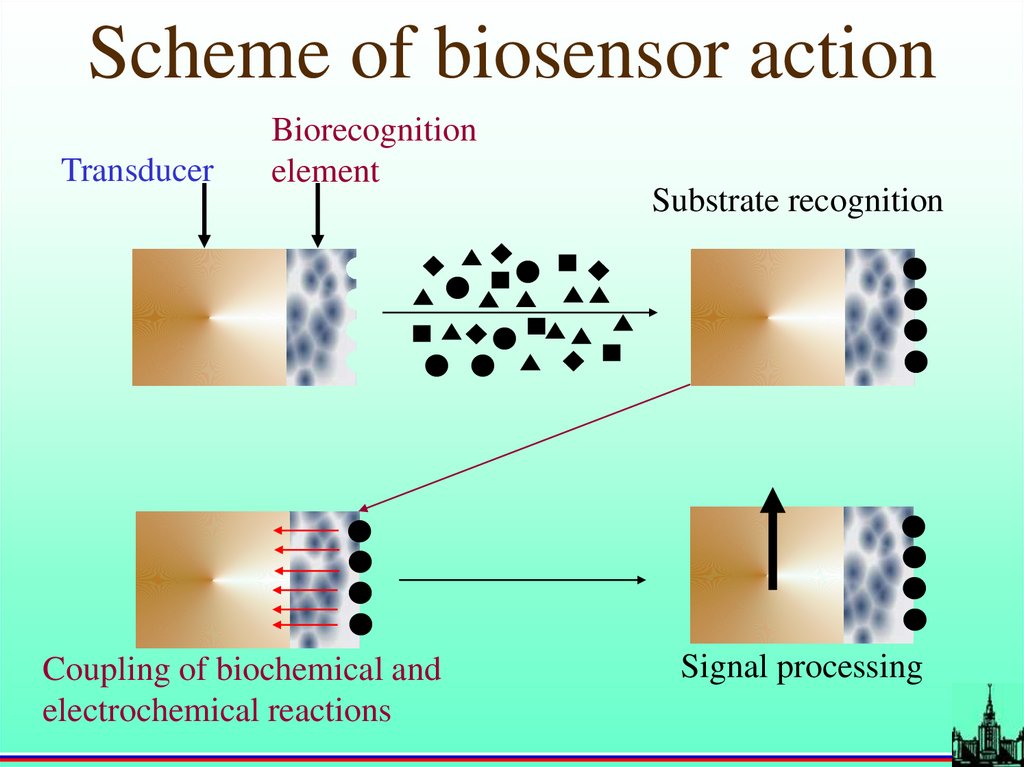
Scheme of biosensor actionTransducer
Biorecognition
element
Coupling of biochemical and
electrochemical reactions
Substrate recognition
Signal processing
3.
Requirements:•detection directly in object without pretreatment;
•a possibility for continuous monitoring;
•a possibility for miniaturization;
•low cost in case of mass production.
4.
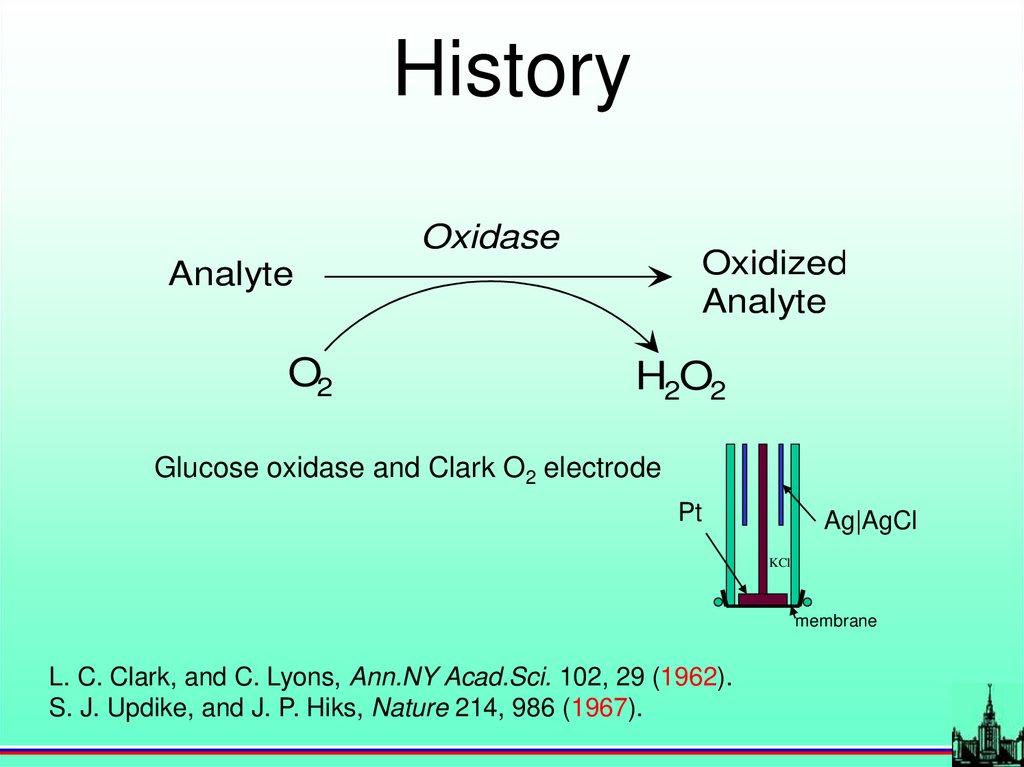
HistoryOxidase
Oxidized
Analyte
Analyte
O2
H2O2
Glucose oxidase and Clark O2 electrode
Pt
Ag|AgCl
KCl
membrane
L. C. Clark, and C. Lyons, Ann.NY Acad.Sci. 102, 29 (1962).
S. J. Updike, and J. P. Hiks, Nature 214, 986 (1967).
5.
ИДЕЯ ФЕРМЕНТНОГО ЭЛЕКТРОДАVolume 102 Issue Automated and
Semi-Automated Systems in Clinical Chemistry , Pages 3 - 180
(October 1962)
A- электрод сравнения
B- рабочий электрод
C- цилиндр
D- электролит
E, G - мембраны
F- фермент
6.
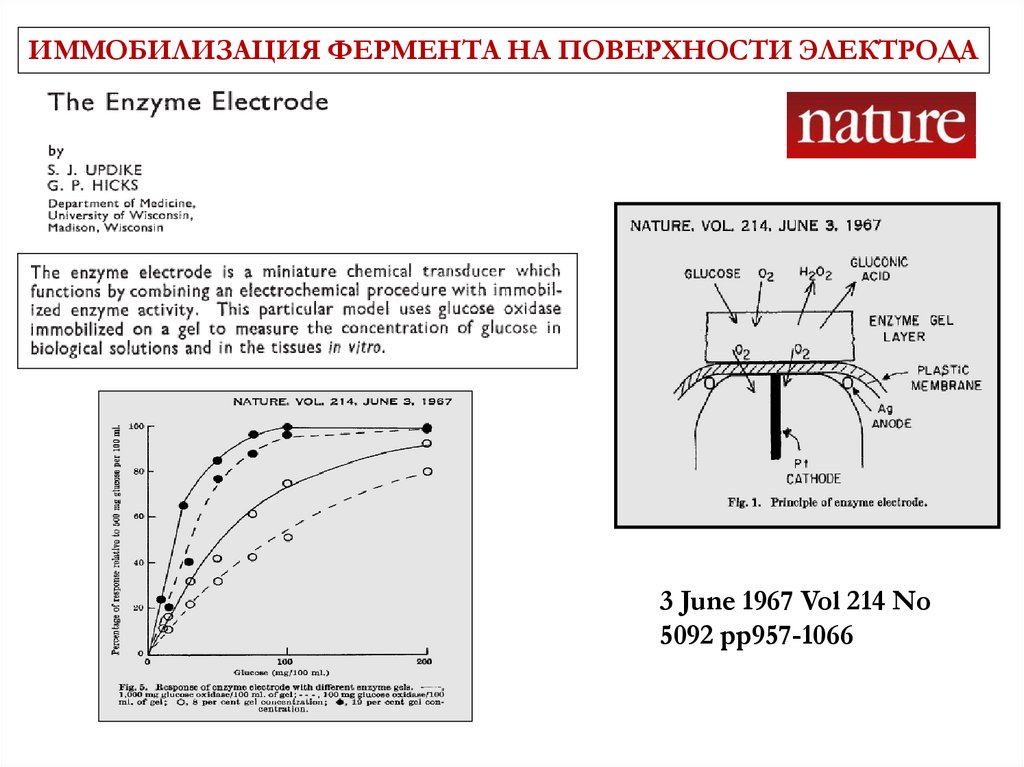
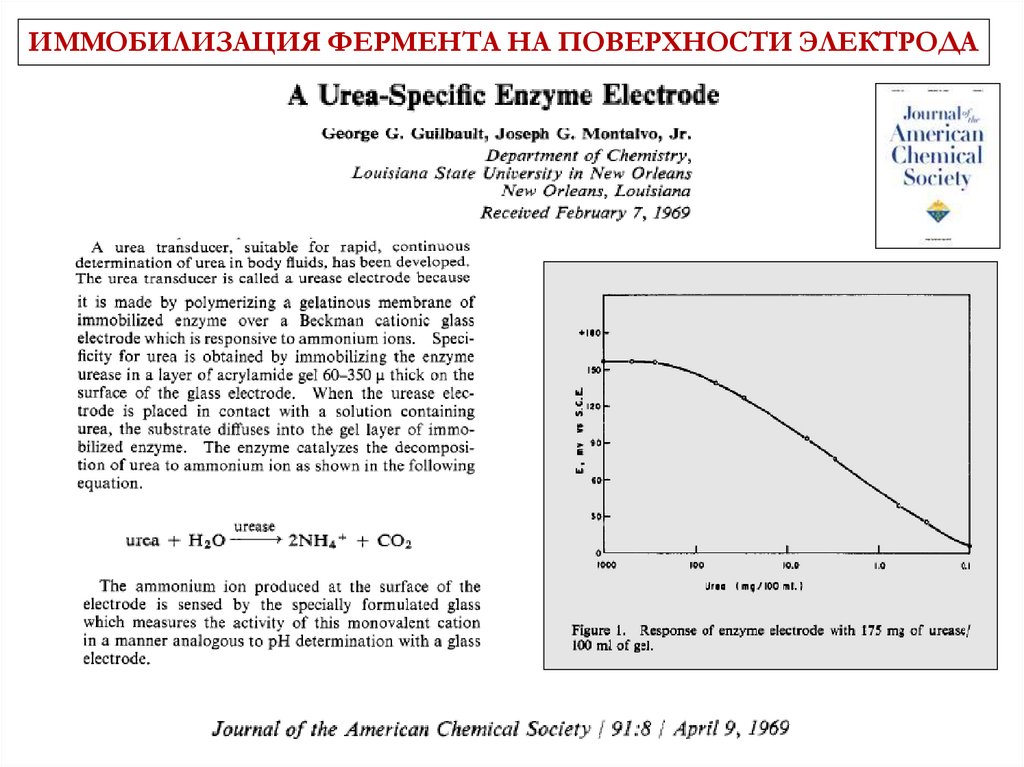
ИММОБИЛИЗАЦИЯ ФЕРМЕНТА НА ПОВЕРХНОСТИ ЭЛЕКТРОДА3 June 1967 Vol 214 No
5092 pp957-1066
7.
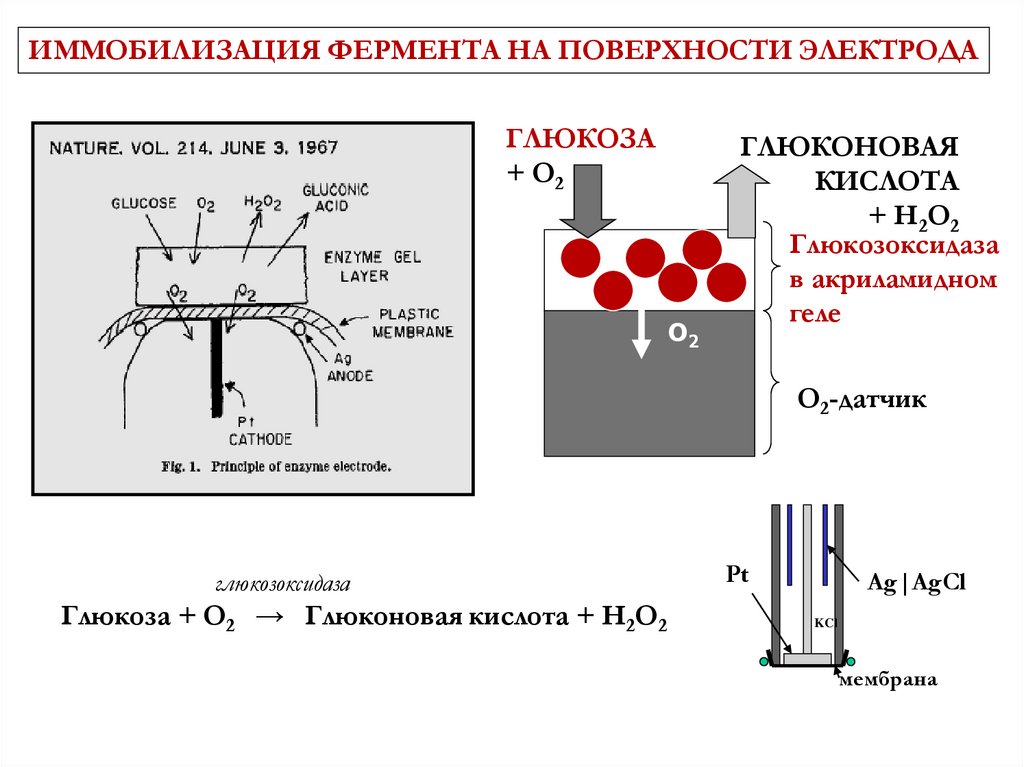
ИММОБИЛИЗАЦИЯ ФЕРМЕНТА НА ПОВЕРХНОСТИ ЭЛЕКТРОДАГЛЮКОЗА
+ O2
O2
ГЛЮКОНОВАЯ
КИСЛОТА
+ H2O2
Глюкозоксидаза
в акриламидном
геле
O2-датчик
глюкозоксидаза
Глюкоза + O2 → Глюконовая кислота + H2O2
Pt
Ag|AgCl
KCl
мембрана
8.
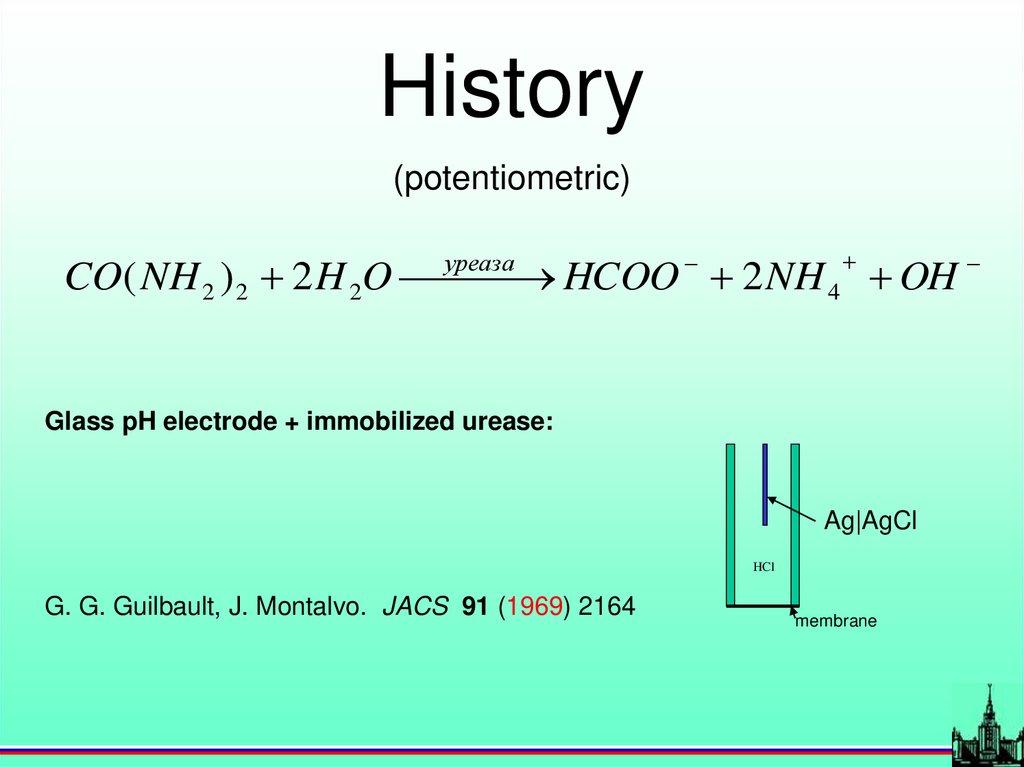
History(potentiometric)
уреаза
CO( NH 2 ) 2 2 H 2O
HCOO 2 NH 4 OH
Glass pH electrode + immobilized urease:
Ag|AgCl
HCl
G. G. Guilbault, J. Montalvo. JACS 91 (1969) 2164
membrane
9.
ИММОБИЛИЗАЦИЯ ФЕРМЕНТА НА ПОВЕРХНОСТИ ЭЛЕКТРОДА10.
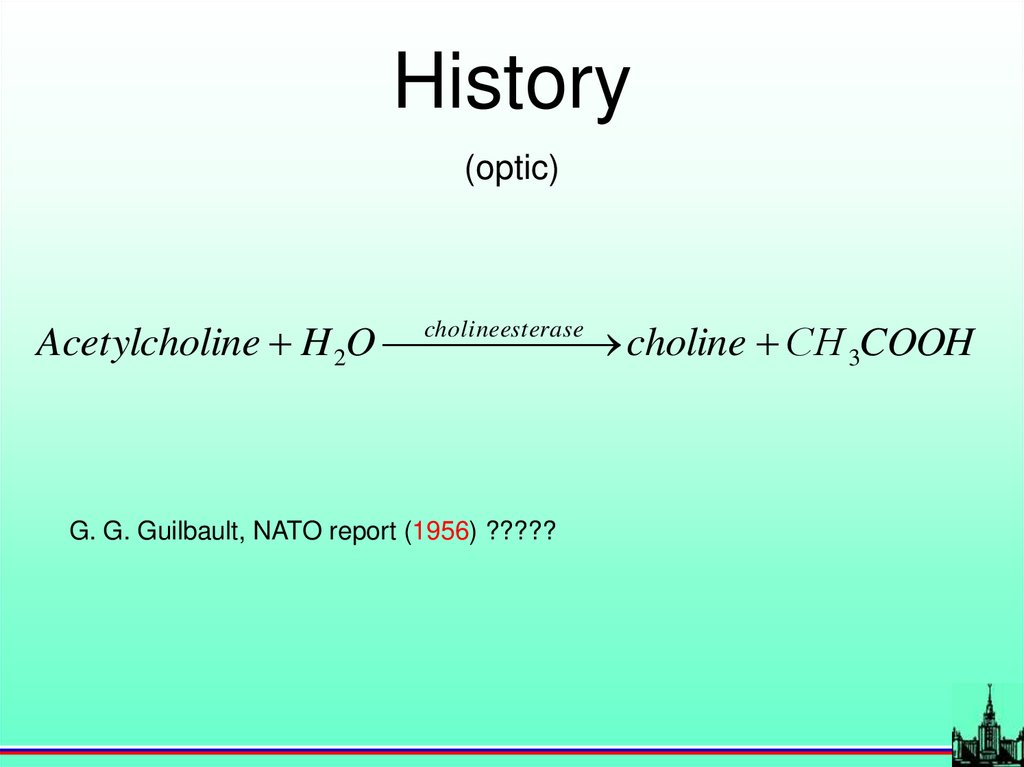
History(optic)
Acetylcholine H 2O choline
esterase
choline СH 3COOH
G. G. Guilbault, NATO report (1956) ?????
11.
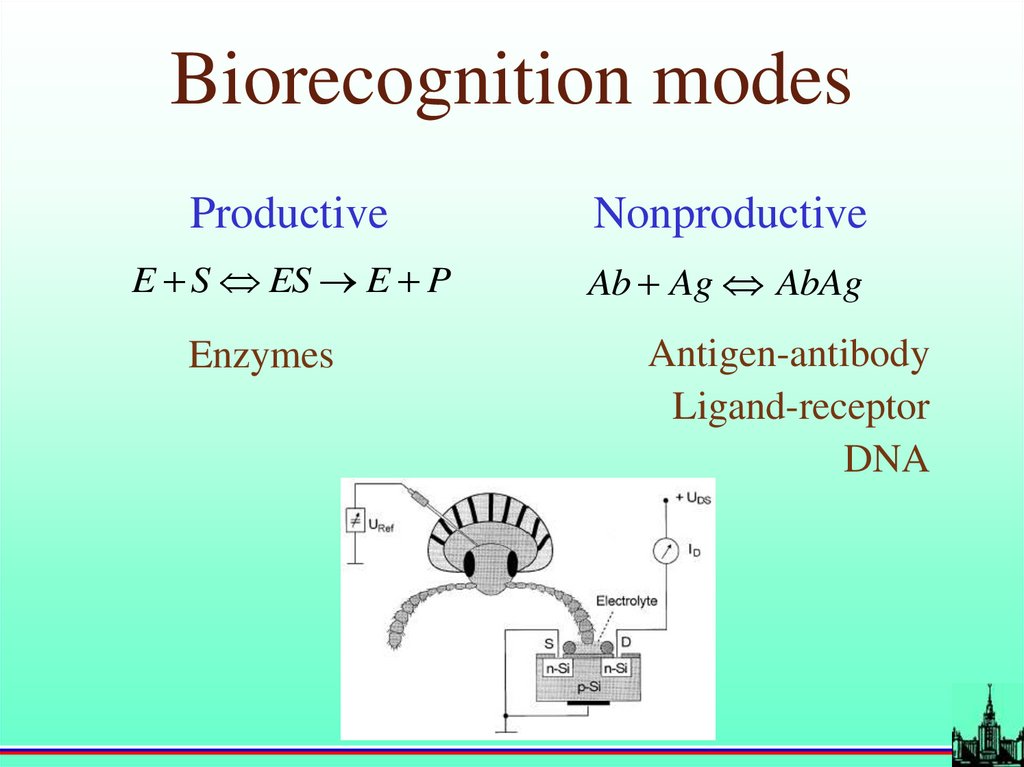
Biorecognition modesProductive
Nonproductive
E S ES E P
Ab Ag AbAg
Enzymes
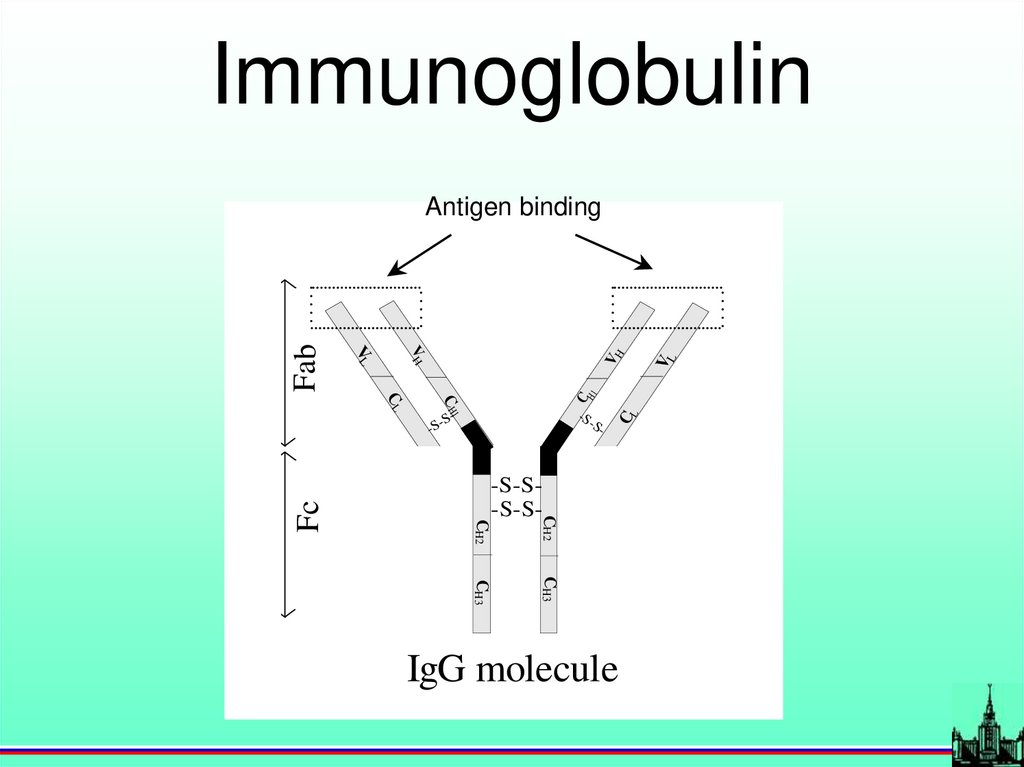
Antigen-antibody
Ligand-receptor
DNA
12.
13.
Immunoglobulin-S
-
S-
S-
CL
-S-
-S-S-S-S-
CH2
CH2
Fc
C
C H1
CL
H1
CH3
CH3
IgG molecule
VL
VH
VH
VL
Fab
Antigen binding
14.
DNA15.
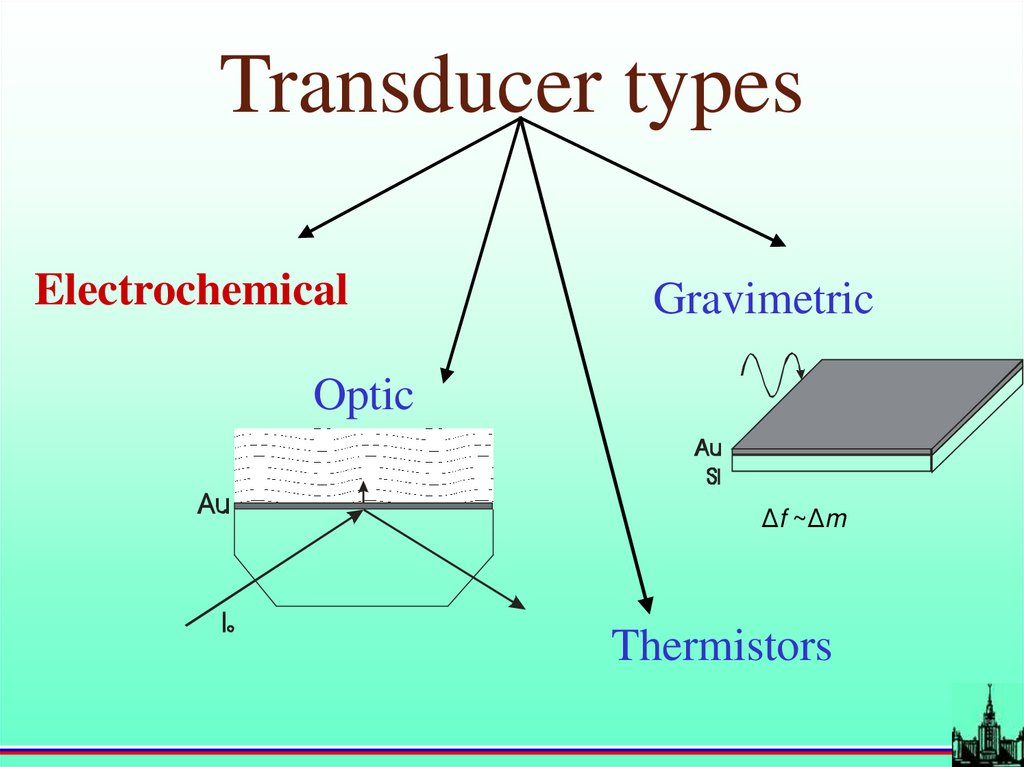
Transducer typesElectrochemical
Gravimetric
Optic
Δf ~Δm
Thermistors
16.
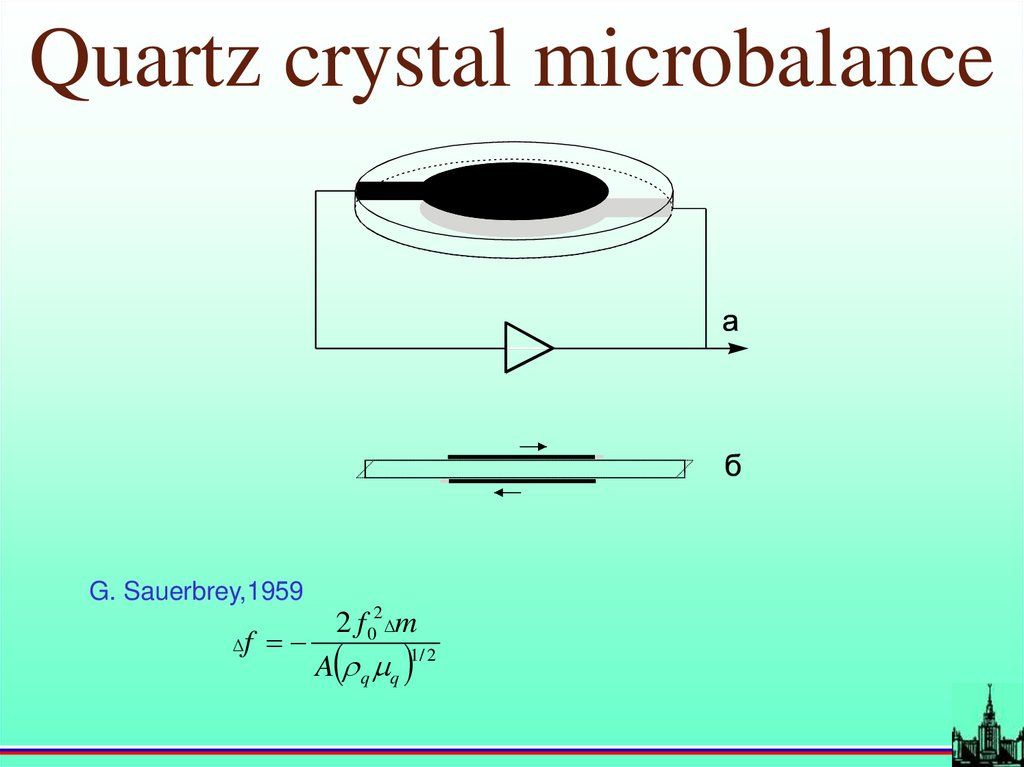
Quartz crystal microbalanceG. Sauerbrey,1959
f
2 f 02 m
A q q
1/ 2
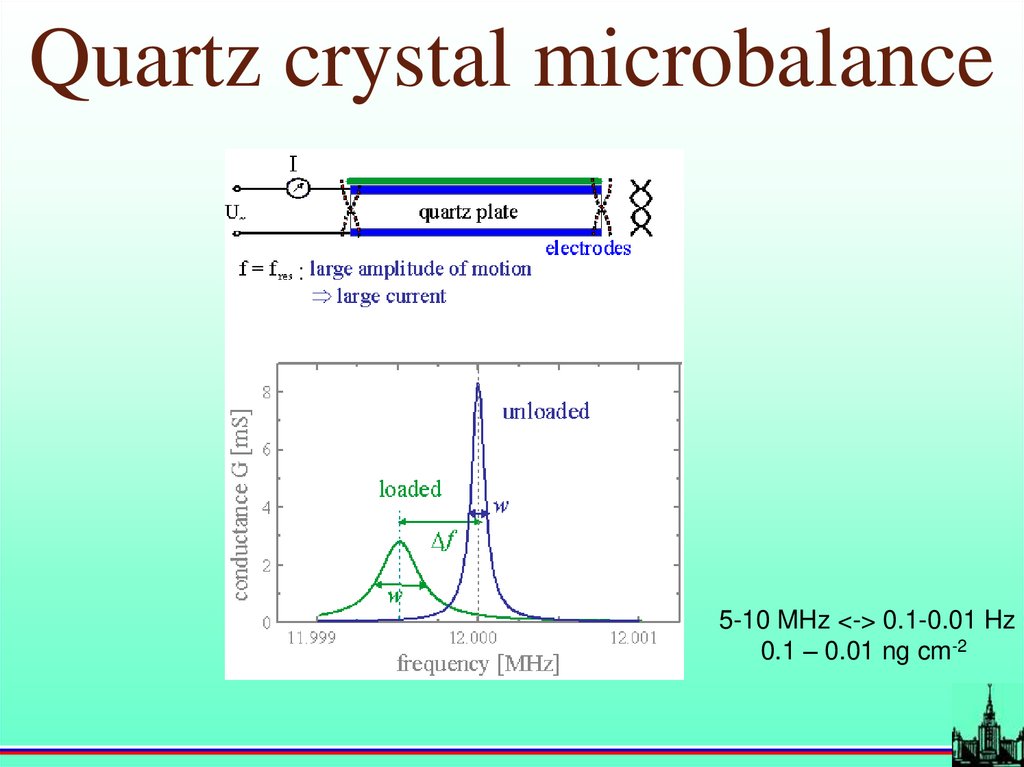
17.
Quartz crystal microbalance5-10 MHz <-> 0.1-0.01 Hz
0.1 – 0.01 ng cm-2
18.
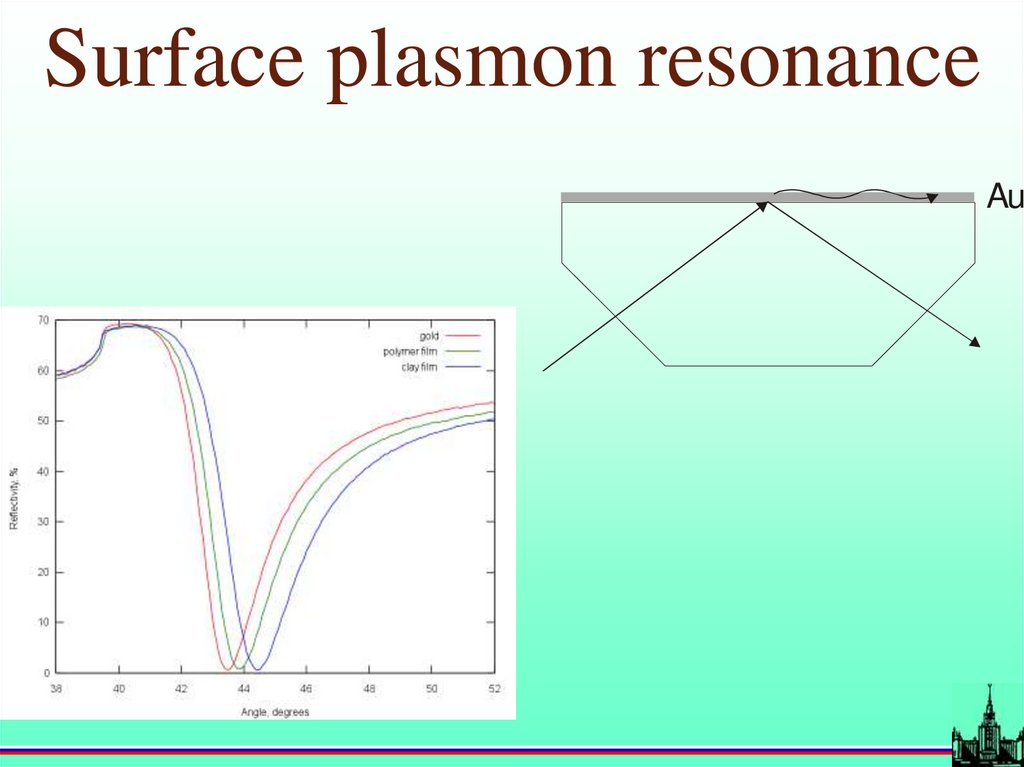
Surface plasmon resonanceAu
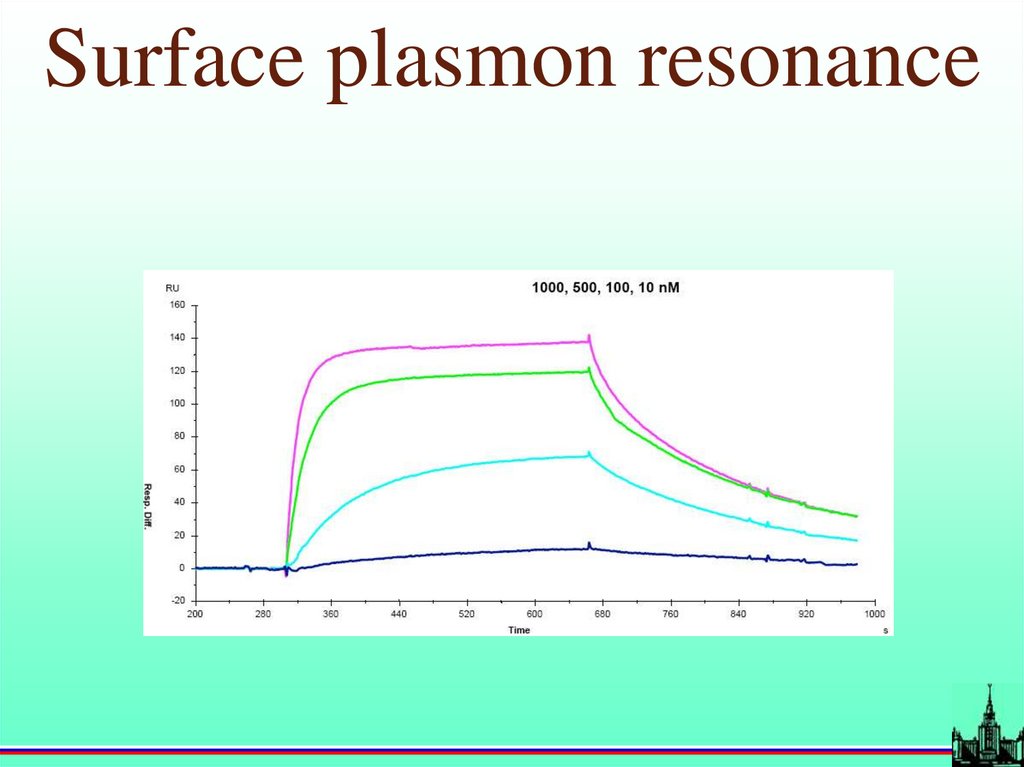
19.
Surface plasmon resonance20.
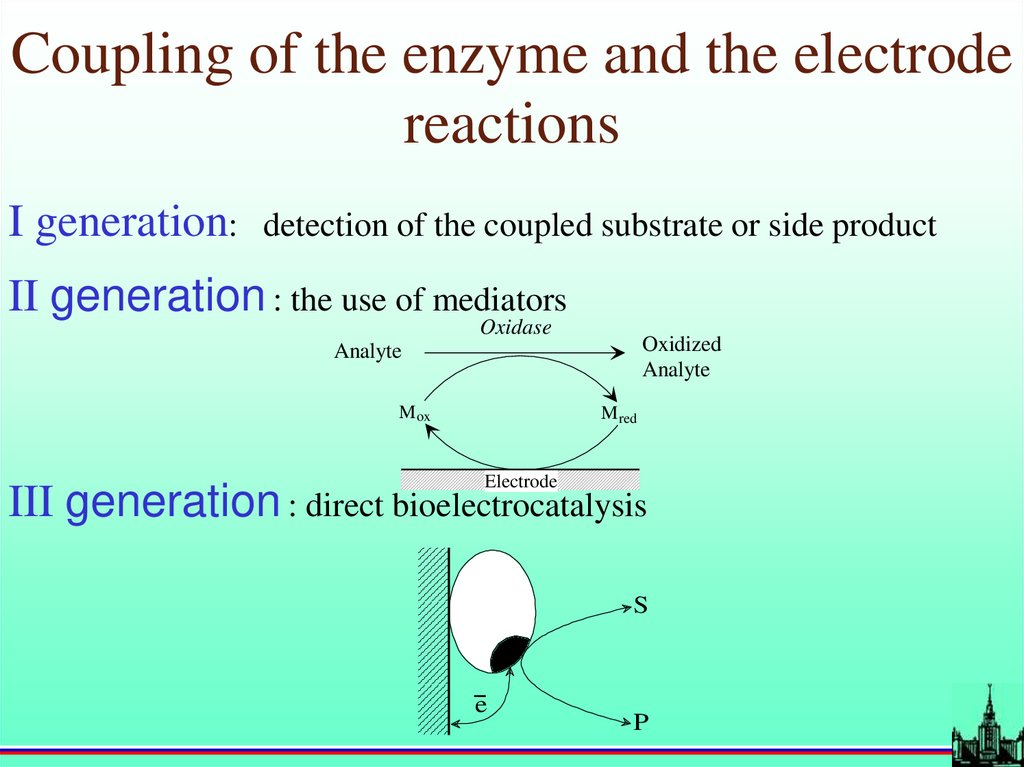
Coupling of the enzyme and the electrodereactions
I generation:
detection of the coupled substrate or side product
II generation : the use of mediators
Oxidase
Oxidized
Analyte
Analyte
M ox
M red
Electrode
III generation : direct bioelectrocatalysis
S
-e
P
21.
Ist generation biosensors(amperometric)
Oxidase
Analyte
O2
Oxidized
Analyte
H2O2
Glucose oxidase and Clark O2 electrode
Pt
Ag|AgCl
KCl
membrane
22.
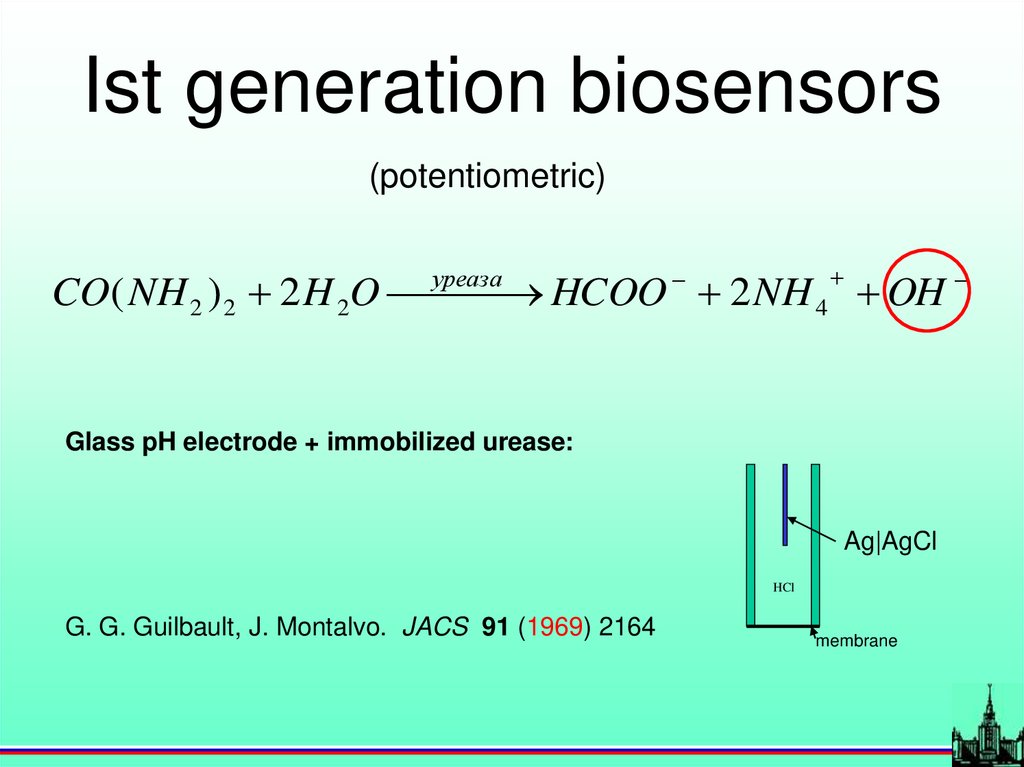
Ist generation biosensors(potentiometric)
уреаза
CO( NH 2 ) 2 2 H 2O
HCOO 2 NH 4 OH
Glass pH electrode + immobilized urease:
Ag|AgCl
HCl
G. G. Guilbault, J. Montalvo. JACS 91 (1969) 2164
membrane
23.
Potentiometric biosensorsUse the enzymes from almost all groups
Enzyme
Product
Substrate
H+
Transducer:
or
H+
Reference
electrode
Uref..
i
• glass Ph electrode
• field effect transistor
• modified electrode
+Udc
insulator
solution
изолятор
Si3 N 4
SiO2
n-Si
n-Si
source
drain
p-Si
24.
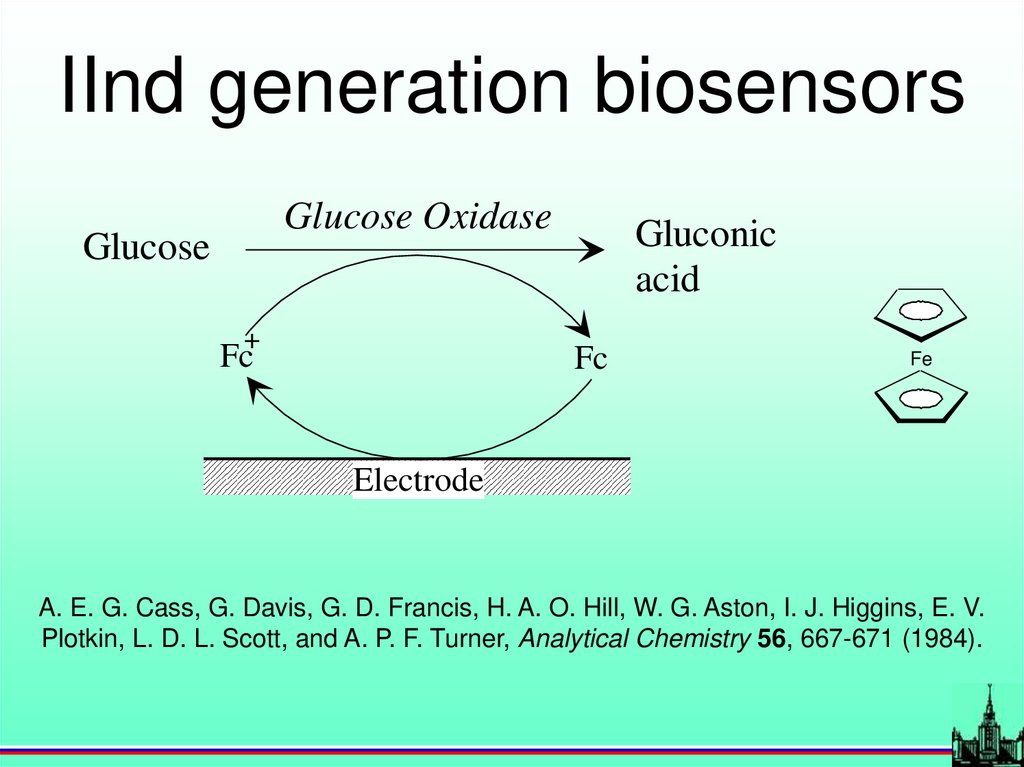
IInd generation biosensorsGlucose Oxidase
Glucose
+
Fc
Gluconic
acid
Fc
Fe
Electrode
A. E. G. Cass, G. Davis, G. D. Francis, H. A. O. Hill, W. G. Aston, I. J. Higgins, E. V.
Plotkin, L. D. L. Scott, and A. P. F. Turner, Analytical Chemistry 56, 667-671 (1984).
25. What Is Diabetes?
Can cause:Blindness
Heart attack
Poor circulation
Gangrene
Kidney
Death
No
dysfunction
cure, but glucose monitoring
can prevent long-term problems
26.
Glucose testsAccu-Chek Complete BG System(Boehringer Mannheim)
Accu-Chek Easy(Boehringer Mannheim)
Accu-Chek Instant(Boehringer Mannheim)
Accu-Chek Instant Plus(Boehringer Mannheim)
Autolet® II Clinisafe(Owen Mumford)
Autolet® Lite Starter Pack(Owen Mumford)
Blood Glucose Strips(Roche)
Exatech®(Medisense)
Fingerstix Lancets(Bayer)
Glucofilm™ Test Strips(Bayer)
Glucose Control Solution(Roche)
Glutose®(Roche)
Lifescan One Touch® Basic™ System(Johnson & Johnson)
Medipoint Blood Lancets(Medipoint)
Monolet Lancet(Kendall-Sherwood)
Soft-Touch® II(Boehringer Mannheim)
Softclix(Roche)
Unilet Long-Body™ Lancets(Owen Mumford)
Unistik™-2(Owen Mumford)
27.
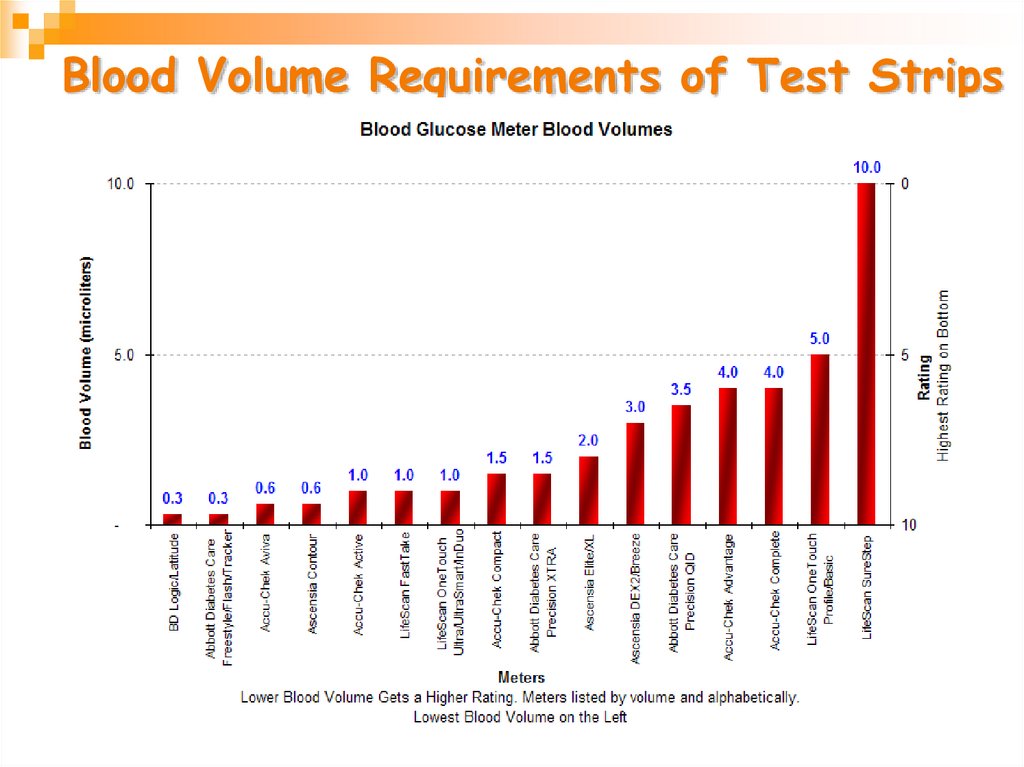
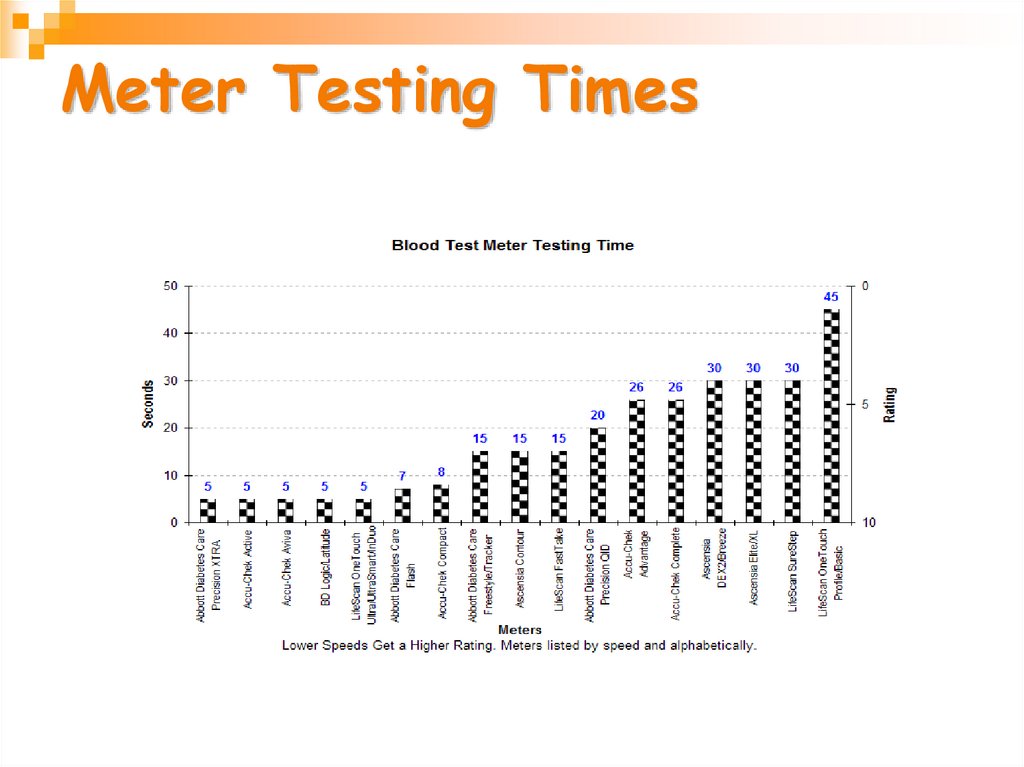
More than 33 different meters arecommercially available from 11 companies.
They differ in several ways including:
•Amount of blood needed for each test
•Testing speed
•Alternative site
•Overall size
•Ability to store test results in memory
•Cost of the meter
•Cost of the test strips used
28. Blood Volume Requirements of Test Strips
29. Meter Testing Times
30.
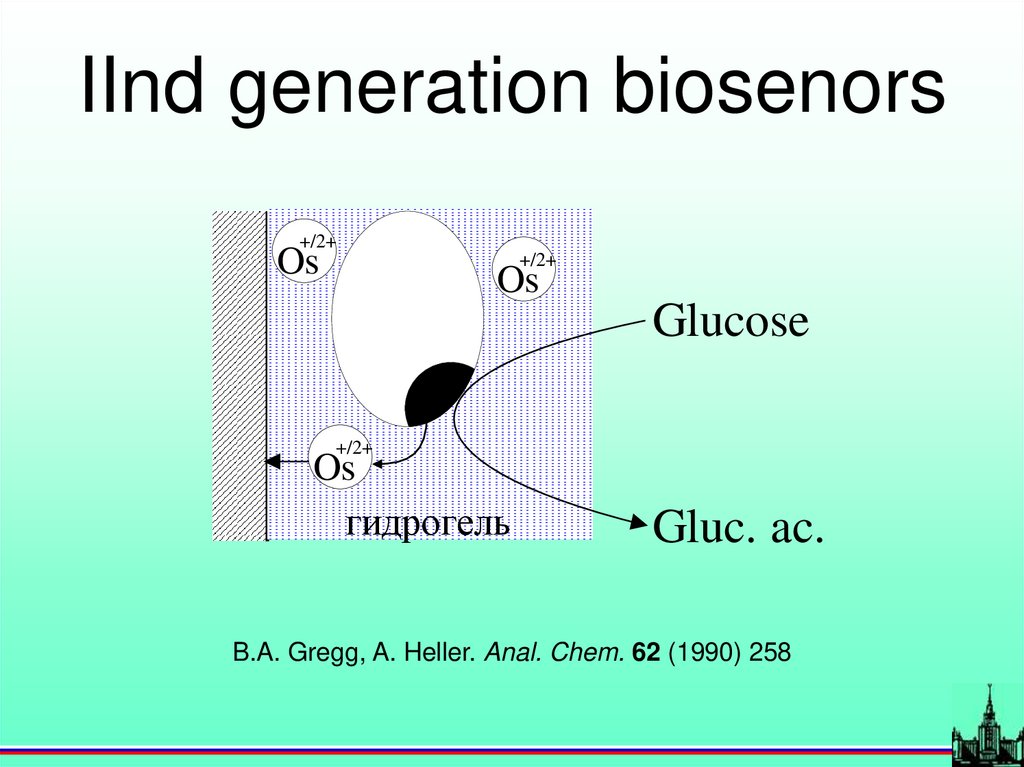
IInd generation biosenors+/2+
Os
+/2+
Os
Glucose
+/2+
Os
гидрогель
Gluc. ac.
B.A. Gregg, A. Heller. Anal. Chem. 62 (1990) 258
31.
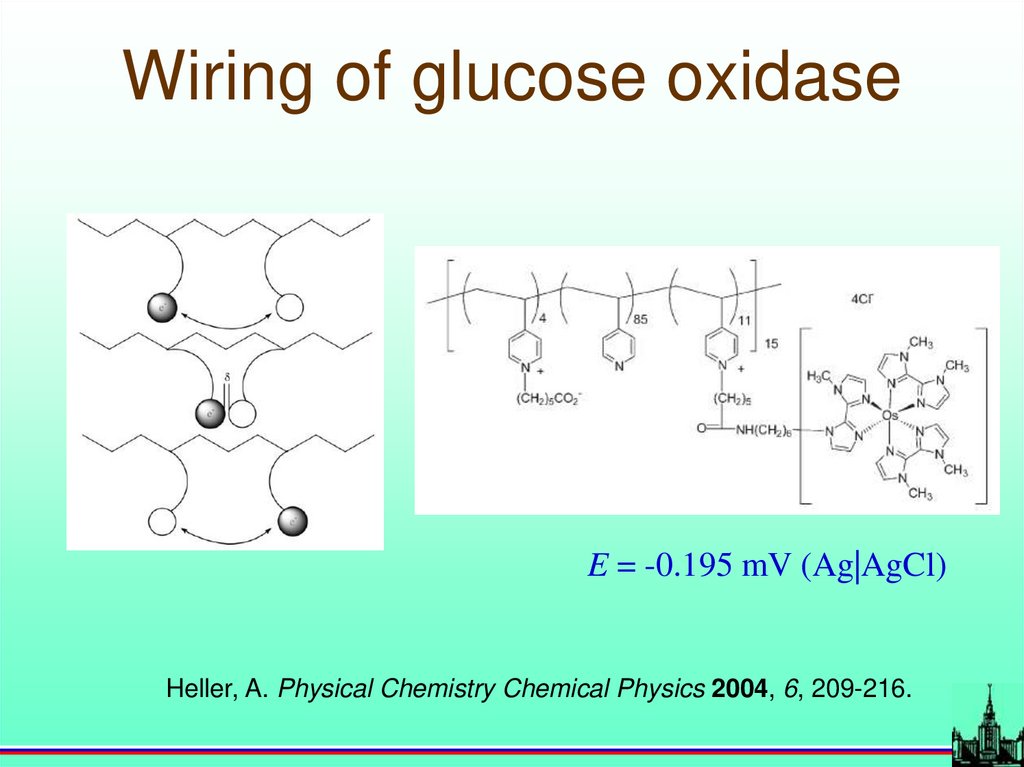
Wiring of glucose oxidaseE = -0.195 mV (Ag|AgCl)
Heller, A. Physical Chemistry Chemical Physics 2004, 6, 209-216.
32.
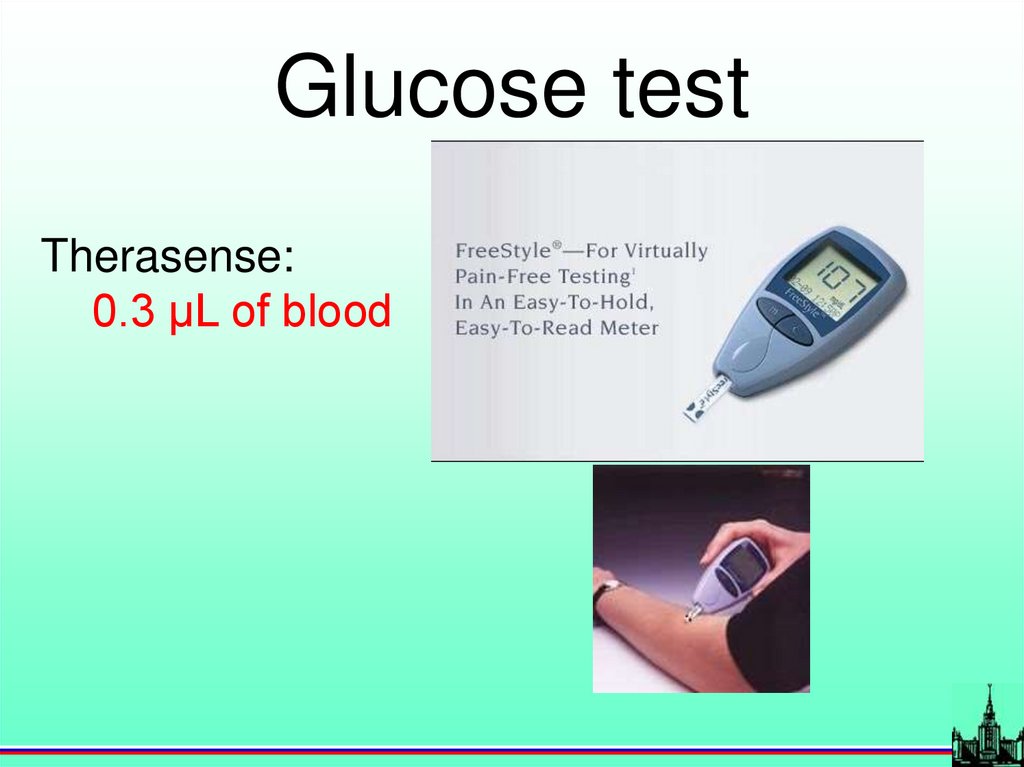
Glucose testTherasense:
0.3 µL of blood
33.
Enzymebioelectrocatalysis
34.
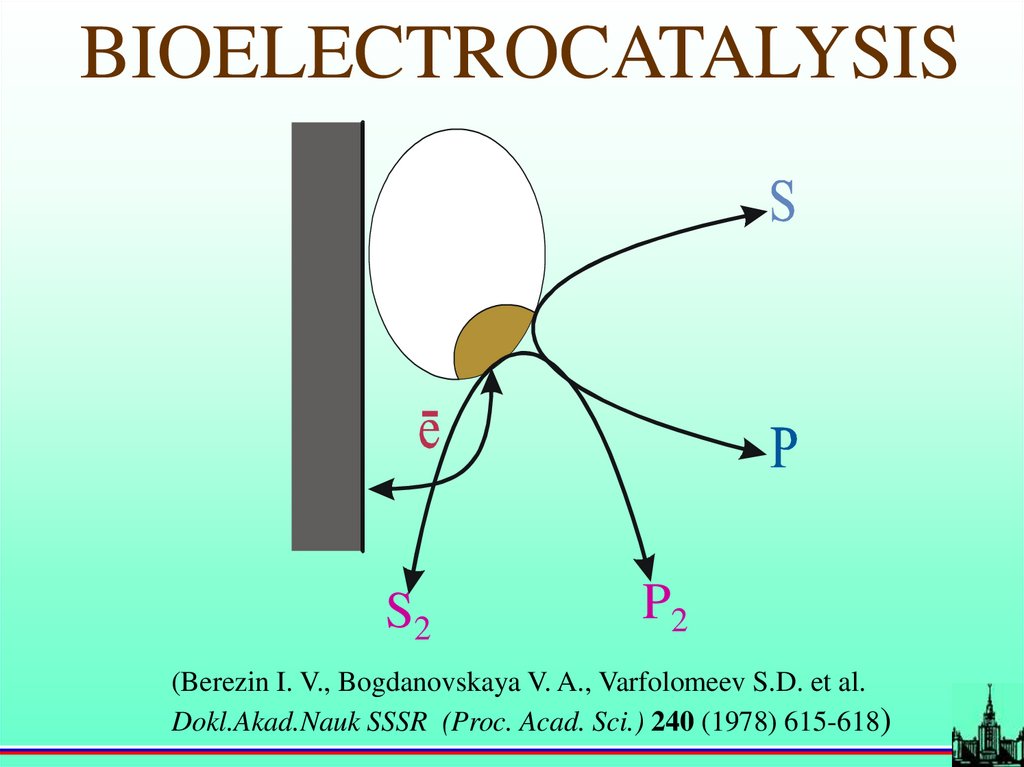
BIOELECTROCATALYSISS2
P2
(Berezin I. V., Bogdanovskaya V. A., Varfolomeev S.D. et al.
Dokl.Akad.Nauk SSSR (Proc. Acad. Sci.) 240 (1978) 615-618)
35.
Direct enzymebioelectrocatalysis
36.
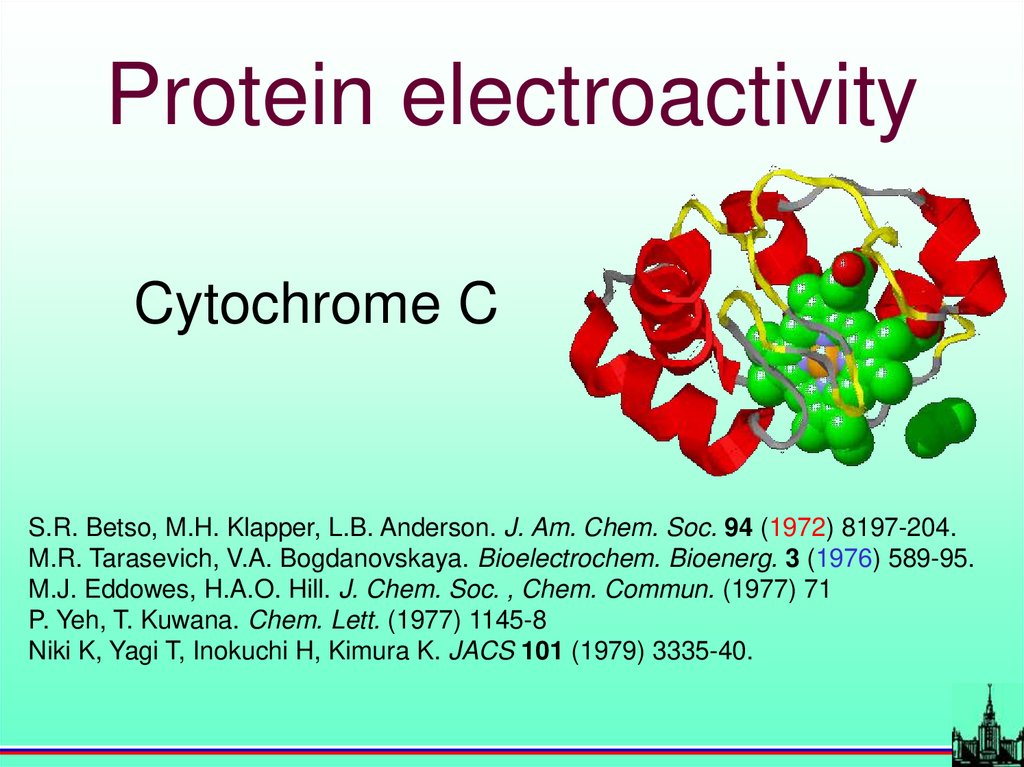
Protein electroactivityCytochrome C
S.R. Betso, M.H. Klapper, L.B. Anderson. J. Am. Chem. Soc. 94 (1972) 8197-204.
M.R. Tarasevich, V.A. Bogdanovskaya. Bioelectrochem. Bioenerg. 3 (1976) 589-95.
M.J. Eddowes, H.A.O. Hill. J. Chem. Soc. , Chem. Commun. (1977) 71
P. Yeh, T. Kuwana. Chem. Lett. (1977) 1145-8
Niki K, Yagi T, Inokuchi H, Kimura K. JACS 101 (1979) 3335-40.
37.
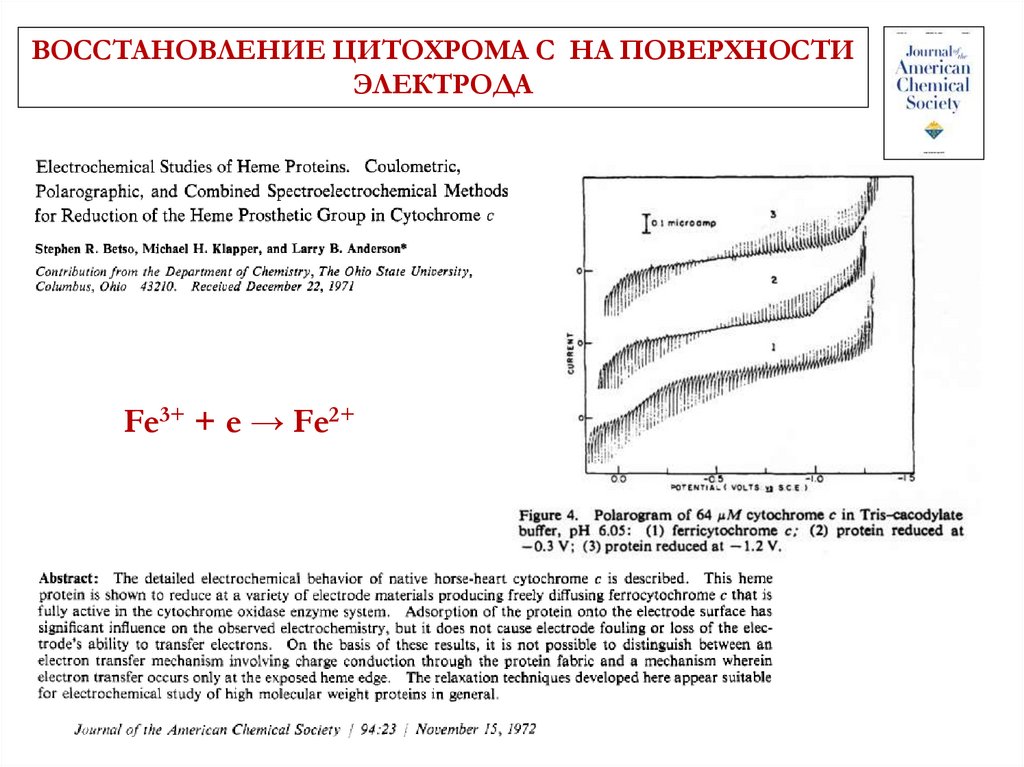
ВОССТАНОВЛЕНИЕ ЦИТОХРОМА С НА ПОВЕРХНОСТИЭЛЕКТРОДА
Fe3+ + e → Fe2+
38.
goldPromoters for protein electroactivity
N
N
ē
ē
M.J. Eddowes, H.A.O. Hill. J. Chem. Soc. , Chem. Commun. (1977) 71
P. Yeh, T. Kuwana. Chem. Lett. (1977) 1145-8
39.
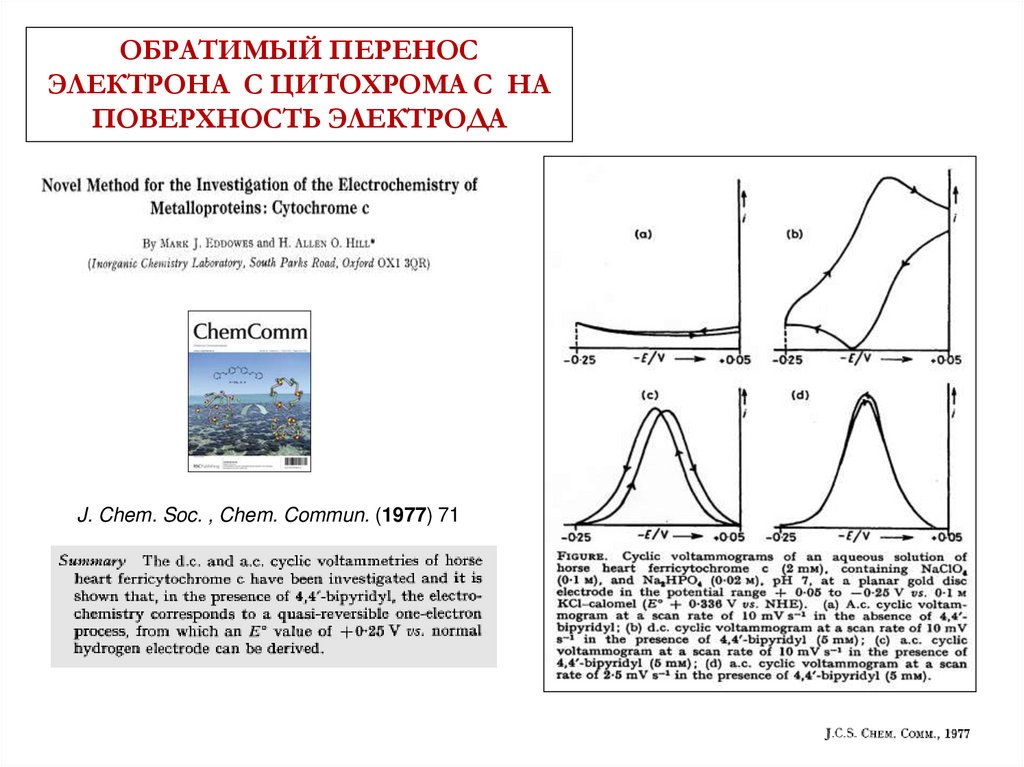
ОБРАТИМЫЙ ПЕРЕНОСЭЛЕКТРОНА С ЦИТОХРОМА С НА
ПОВЕРХНОСТЬ ЭЛЕКТРОДА
40.
ОБРАТИМЫЙ ПЕРЕНОСЭЛЕКТРОНА С ЦИТОХРОМА С НА
ПОВЕРХНОСТЬ ЭЛЕКТРОДА
J. Chem. Soc. , Chem. Commun. (1977) 71
41.
Direct bioelectrocatalysisO2 4 H 4e 2 H 2O
Laccase
Est = 1.2 V
Berezin I. V., Bogdanovskaya V. A., Varfolomeev S.D., M.R. Tarasevich, A.I Yaropolov.
Dokl.Akad.Nauk SSSR (Proc. Acad. Sci.) 240 (1978) 615-618
42.
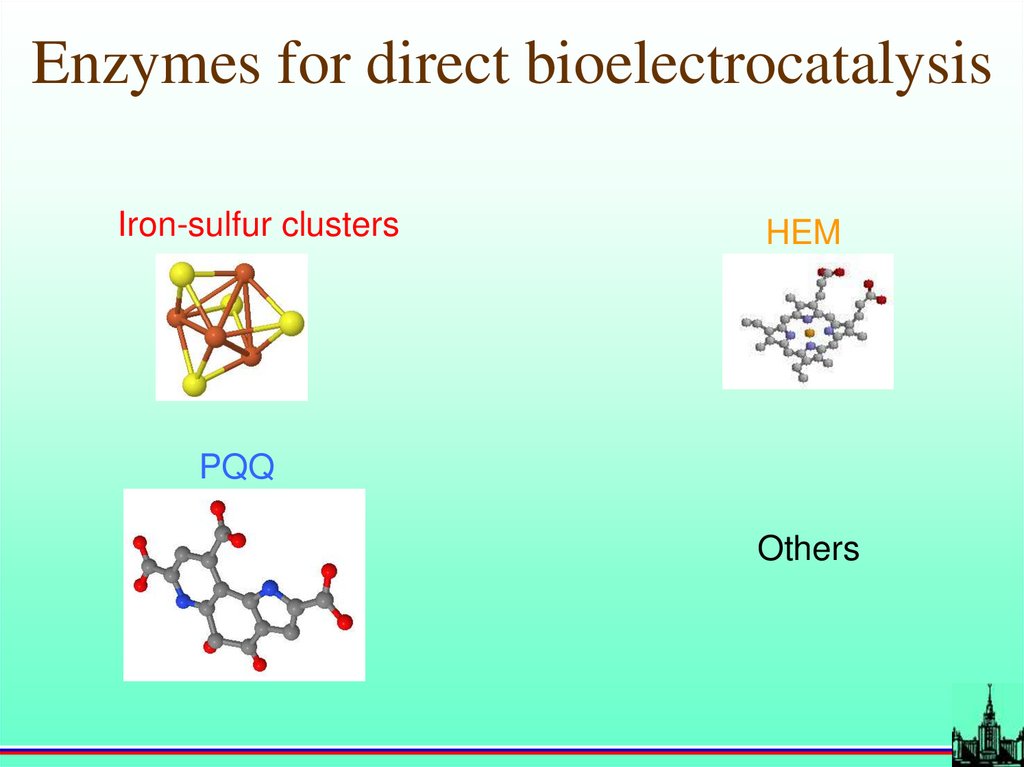
Enzymes for direct bioelectrocatalysisIron-sulfur clusters
HEM
PQQ
Others
43.
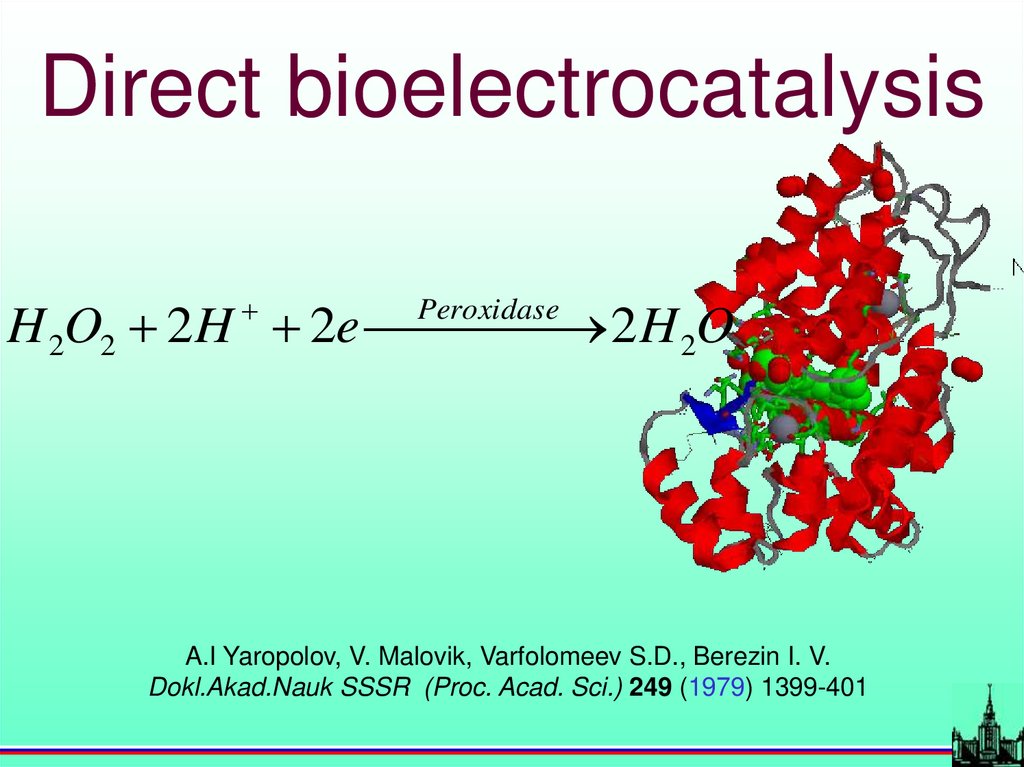
Direct bioelectrocatalysisH 2O2 2 H 2e 2 H 2O
Peroxidase
A.I Yaropolov, V. Malovik, Varfolomeev S.D., Berezin I. V.
Dokl.Akad.Nauk SSSR (Proc. Acad. Sci.) 249 (1979) 1399-401
44.
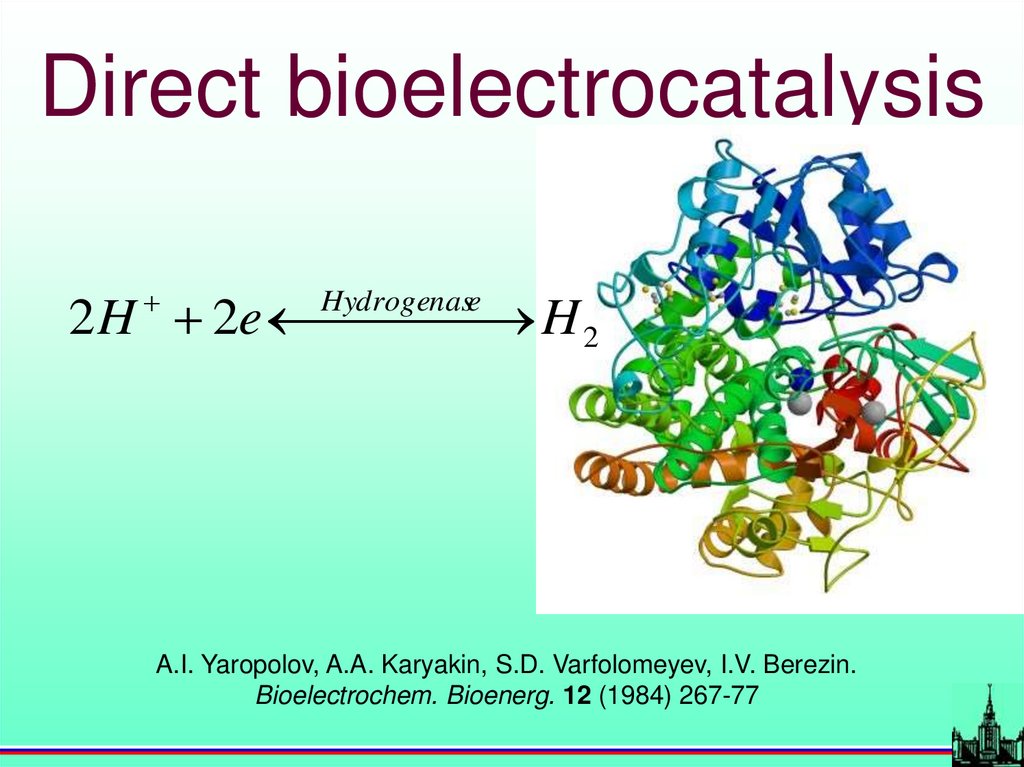
Direct bioelectrocatalysis2 H 2e
H 2
Hydrogenase
A.I. Yaropolov, A.A. Karyakin, S.D. Varfolomeyev, I.V. Berezin.
Bioelectrochem. Bioenerg. 12 (1984) 267-77
45.
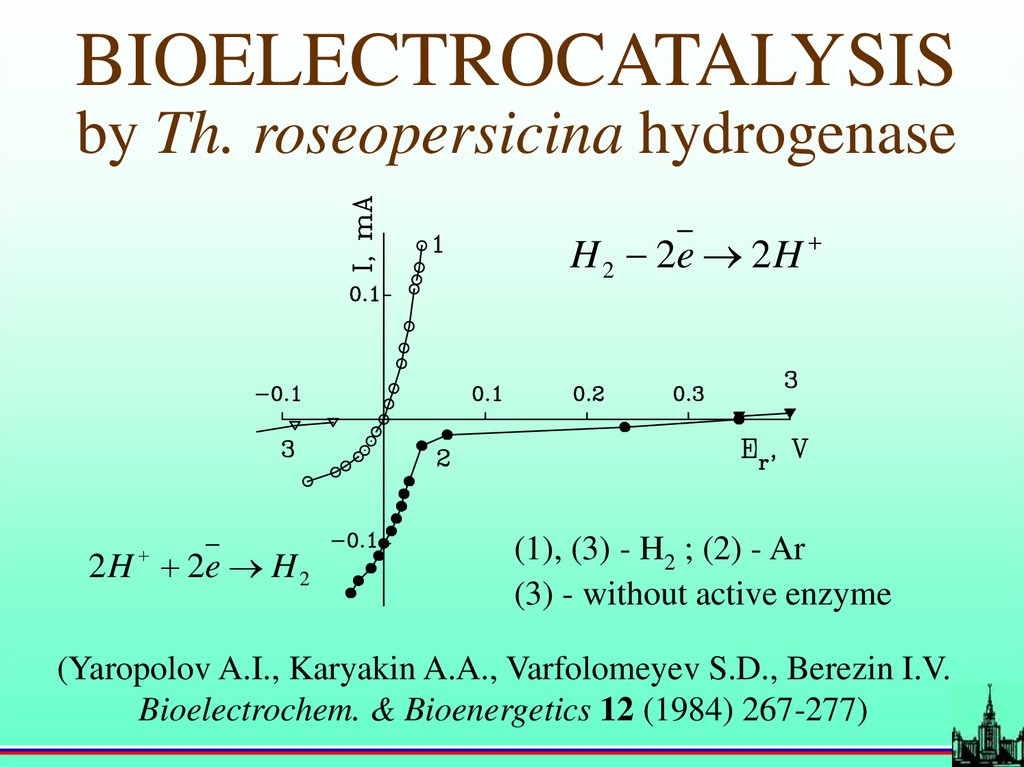
BIOELECTROCATALYSISby Th. roseopersicina hydrogenase
H 2 2e 2 H
2 H 2e H 2
(1), (3) - H2 ; (2) - Ar
(3) - without active enzyme
(Yaropolov A.I., Karyakin A.A., Varfolomeyev S.D., Berezin I.V.
Bioelectrochem. & Bioenergetics 12 (1984) 267-277)
46.
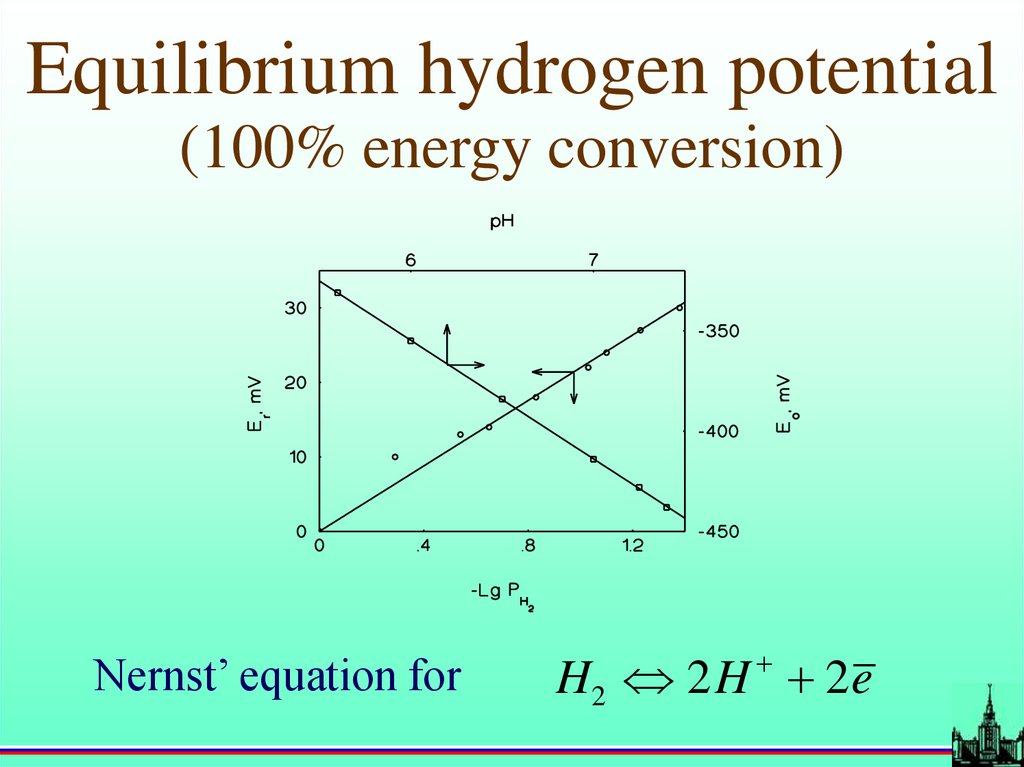
Equilibrium hydrogen potential(100% energy conversion)
Nernst’ equation for
H2 2 H 2 e
47.
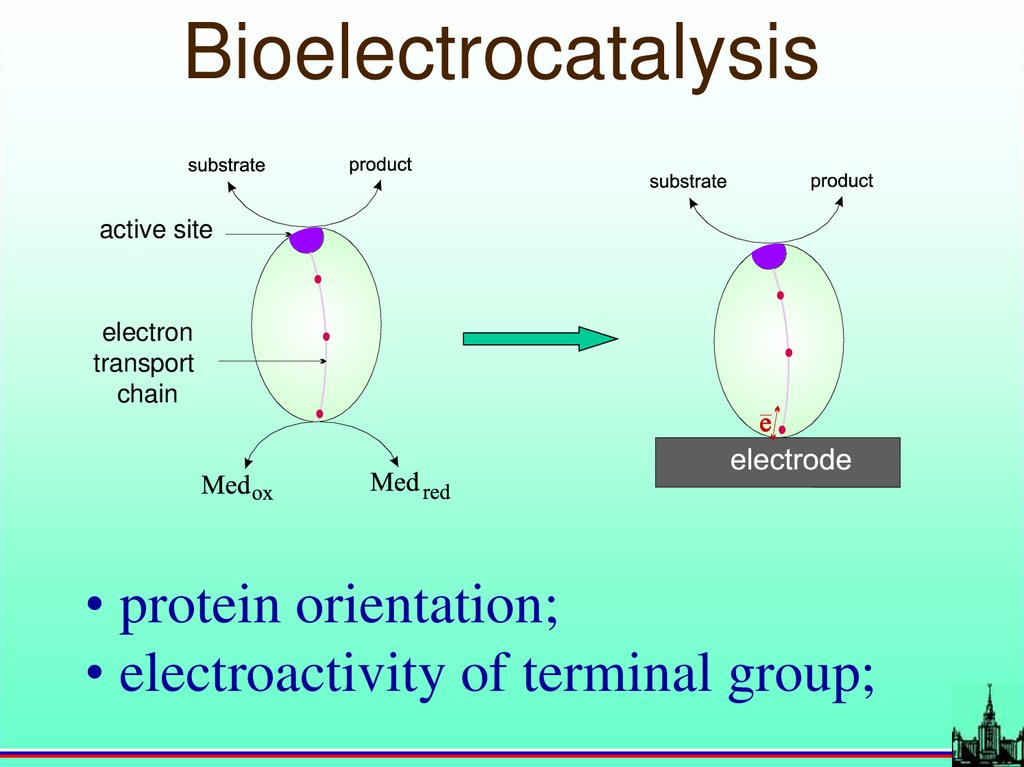
Bioelectrocatalysisactive site
electron
transport
chain
• protein orientation;
• electroactivity of terminal group;
48.
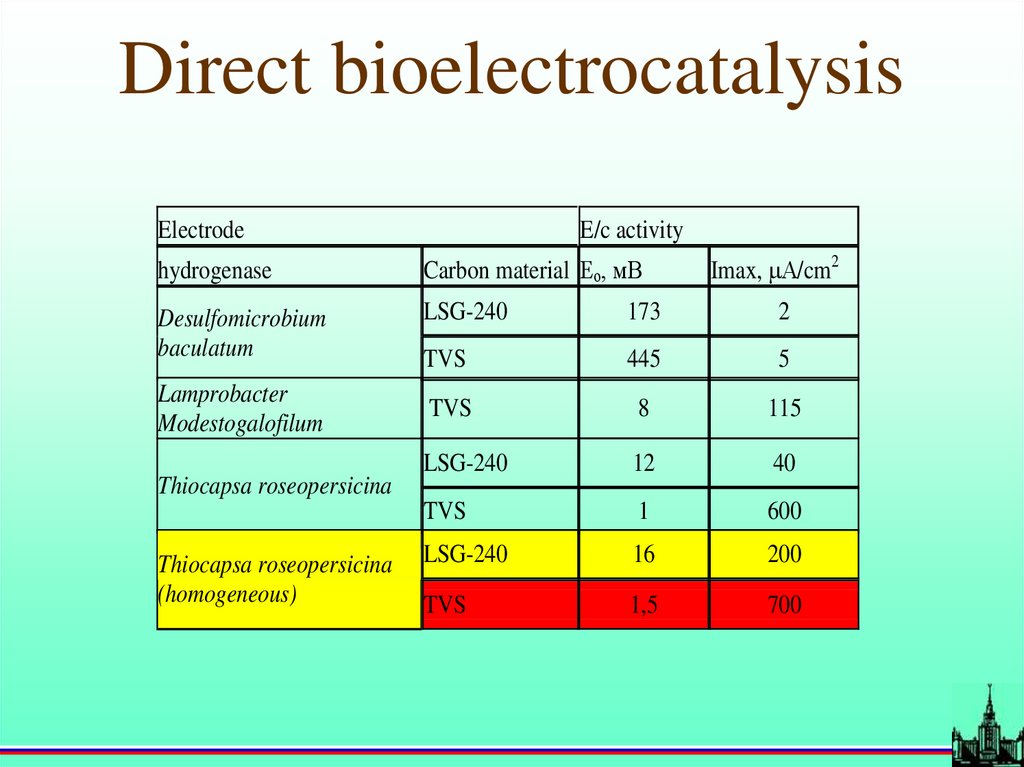
Direct bioelectrocatalysisElectrode
E/c activity
Imax, А/cm2
hydrogenase
Carbon material Ео, мВ
Desulfomicrobium
baculatum
LSG-240
173
2
TVS
445
5
TVS
8
115
LSG-240
12
40
TVS
1
600
LSG-240
16
200
TVS
1,5
700
Lamprobacter
Modestogalofilum
Thiocapsa roseopersicina
Thiocapsa roseopersicina
(homogeneous)
49.
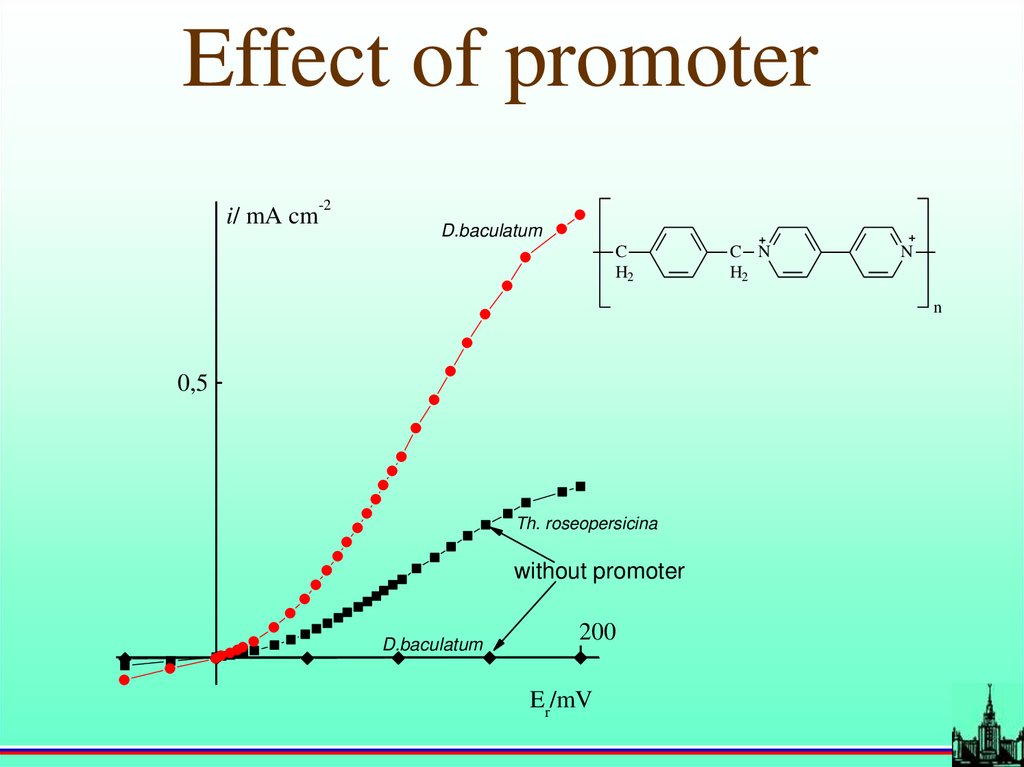
Effect of promoter-2
i/ mA cm
D.baculatum
C
H2
C N
H2
N
n
0,5
Th. roseopersicina
without promoter
D.baculatum
200
Er/mV
50.
Cellobiose dehydrogenase из Myriococcumthermophilum
Km, µM (*)
(kcат/Km)/(kcат/Km)lactose
Lactose
55.3 ± 0.8
1
Cellobiose
26.9 ± 1.6
1.75
Maltose
(2.80 ± 0.08)*103
3.5*10-3
Glucose
2.4*105 ± 1.5*103
5.8*10-4
51.
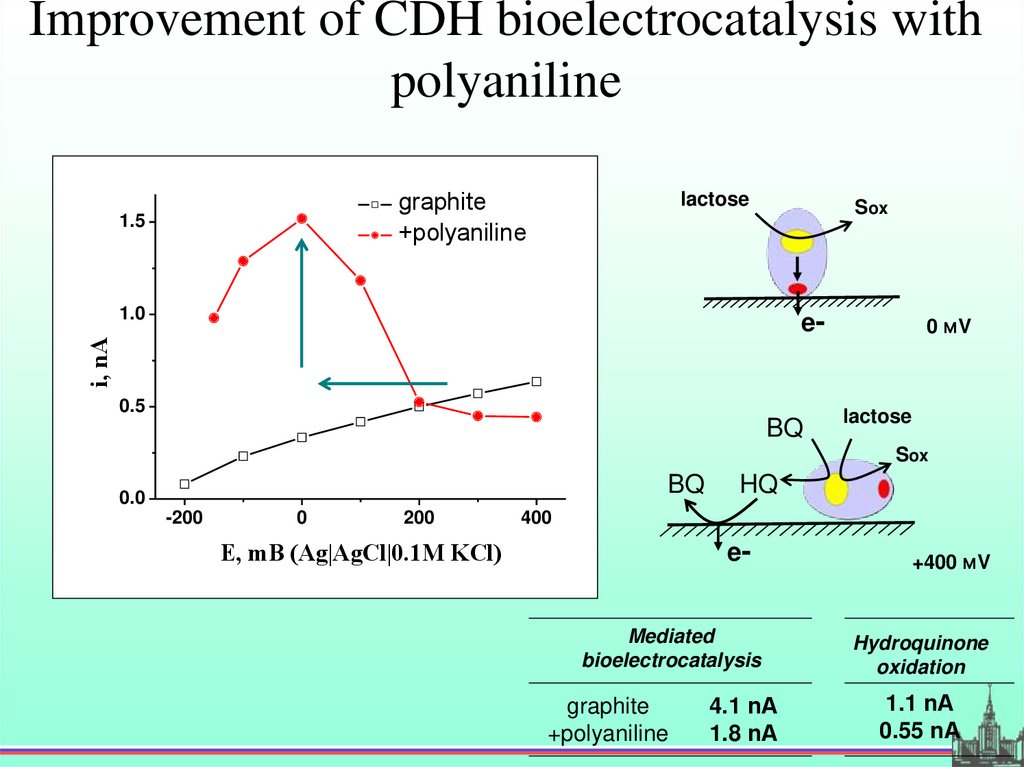
Improvement of CDH bioelectrocatalysis withpolyaniline
graphite
+polyaniline
1.5
lactose
Sox
1.0
i, nA
e-
0.5
BQ
0 мV
lactose
Sox
BQ
0.0
-200
0
200
HQ
400
E, mВ (Ag|AgCl|0.1М KCl)
eMediated
bioelectrocatalysis
graphite
+polyaniline
4.1 nА
1.8 nА
+400 мV
Hydroquinone
oxidation
1.1 nА
0.55 nА
52.
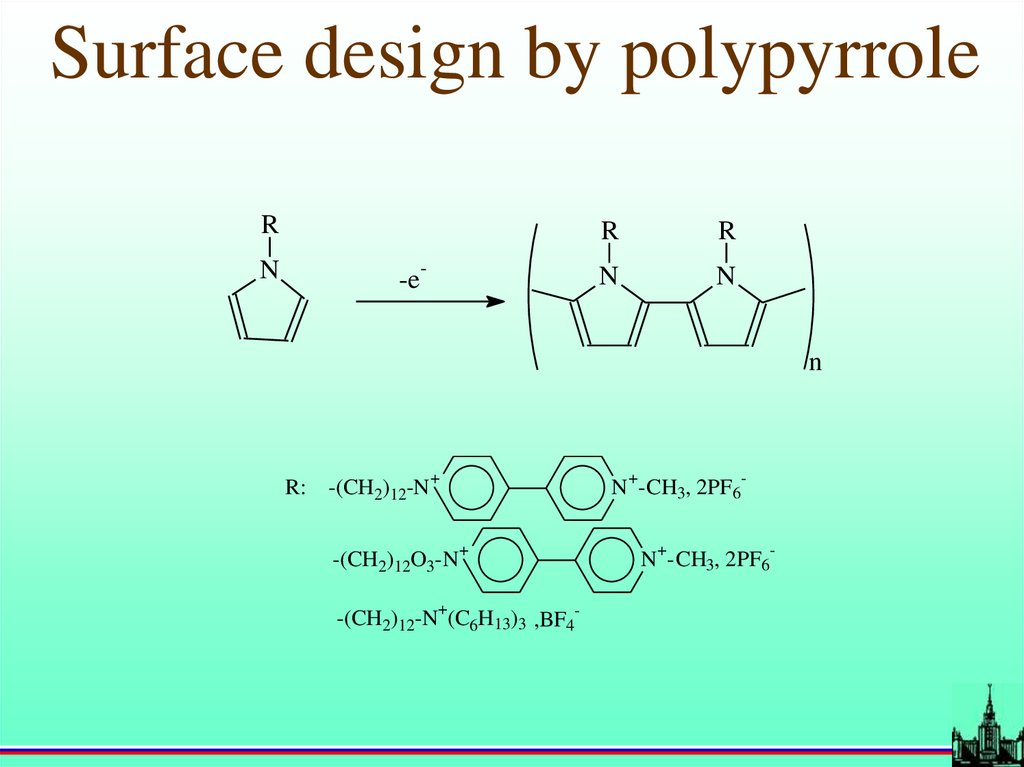
Surface design by polypyrroleR
N
-
-e
R
R
N
N
n
R: -(CH2)12-N
+
-(CH2)12O3-N
+
+
-
N -CH3, 2PF6
+
-(CH2)12-N (C6H13)3 ,BF4-
+
N -CH3, 2PF6
-
53.
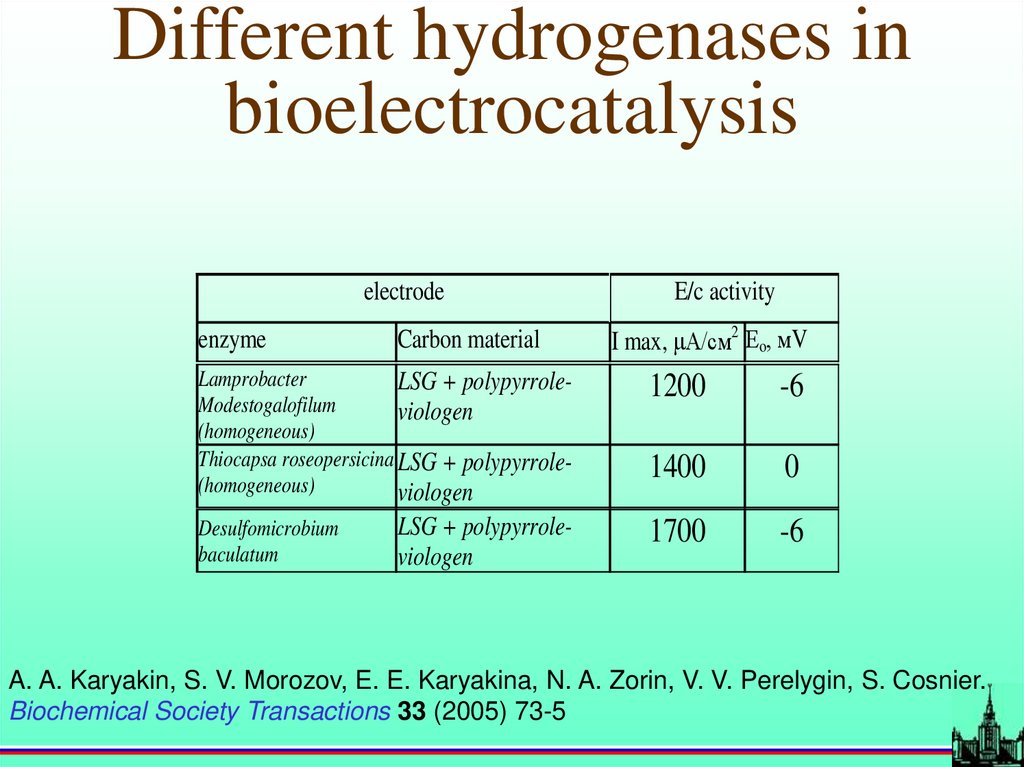
Different hydrogenases inbioelectrocatalysis
electrode
enzyme
Carbon material
E/c activity
I max, А/см2 Еo, мV
Lamprobacter
LSG + polypyrroleModestogalofilum
viologen
(homogeneous)
Thiocapsa roseopersicina LSG + polypyrrole(homogeneous)
viologen
1200
-6
1400
0
LSG + polypyrroleviologen
1700
-6
Desulfomicrobium
baculatum
A. A. Karyakin, S. V. Morozov, E. E. Karyakina, N. A. Zorin, V. V. Perelygin, S. Cosnier.
Biochemical Society Transactions 33 (2005) 73-5
54.
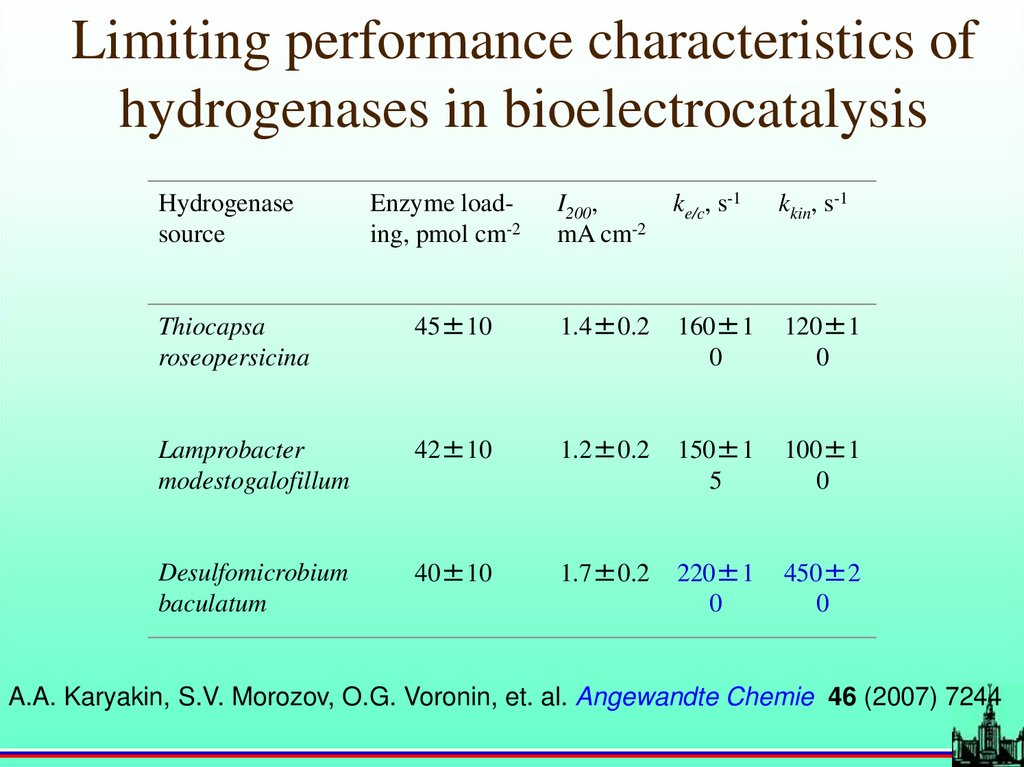
Limiting performance characteristics ofhydrogenases in bioelectrocatalysis
Hydrogenase
source
Enzyme loading, pmol cm-2
I200,
mA cm-2
ke/c, s-1
kkin, s-1
Thiocapsa
roseopersicina
45±10
1.4±0.2
160±1
0
120±1
0
Lamprobacter
modestogalofillum
42±10
1.2±0.2
150±1
5
100±1
0
Desulfomicrobium
baculatum
40±10
1.7±0.2
220±1
0
450±2
0
A.A. Karyakin, S.V. Morozov, O.G. Voronin, et. al. Angewandte Chemie 46 (2007) 7244
55.
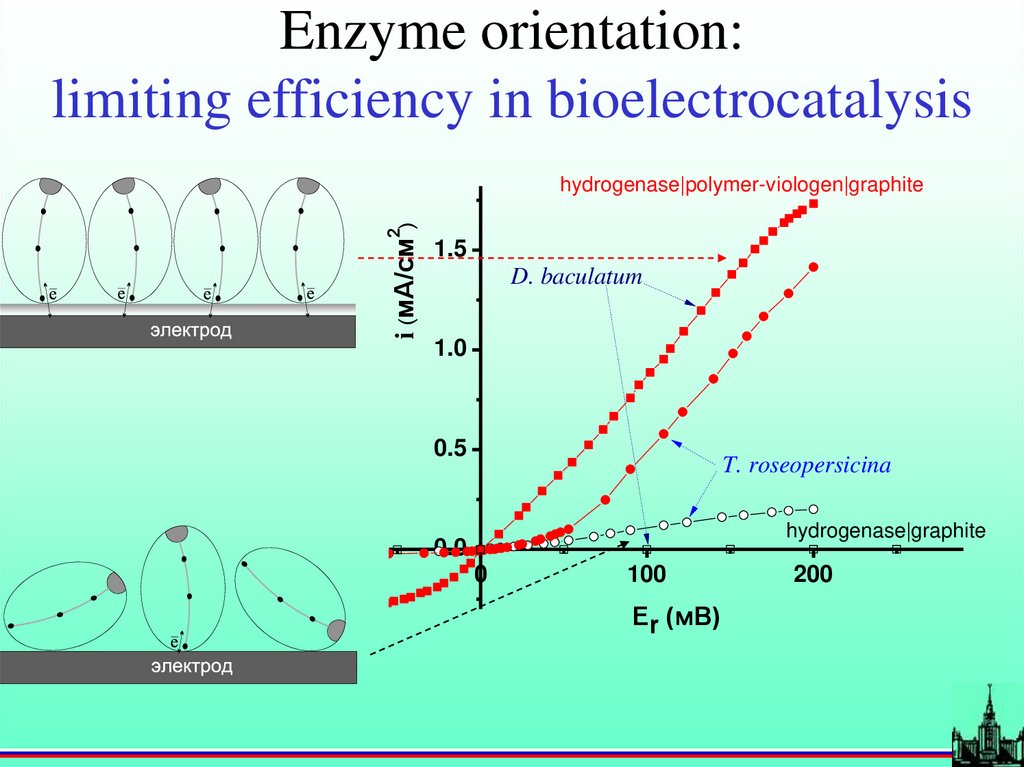
Enzyme orientation:limiting efficiency in bioelectrocatalysis
i (мА/см2)
hydrogenase|polymer-viologen|graphite
1.5
D. baculatum
1.0
0.5
T. roseopersicina
hydrogenase|graphite
0.0
0
100
Er (мВ)
200
56.
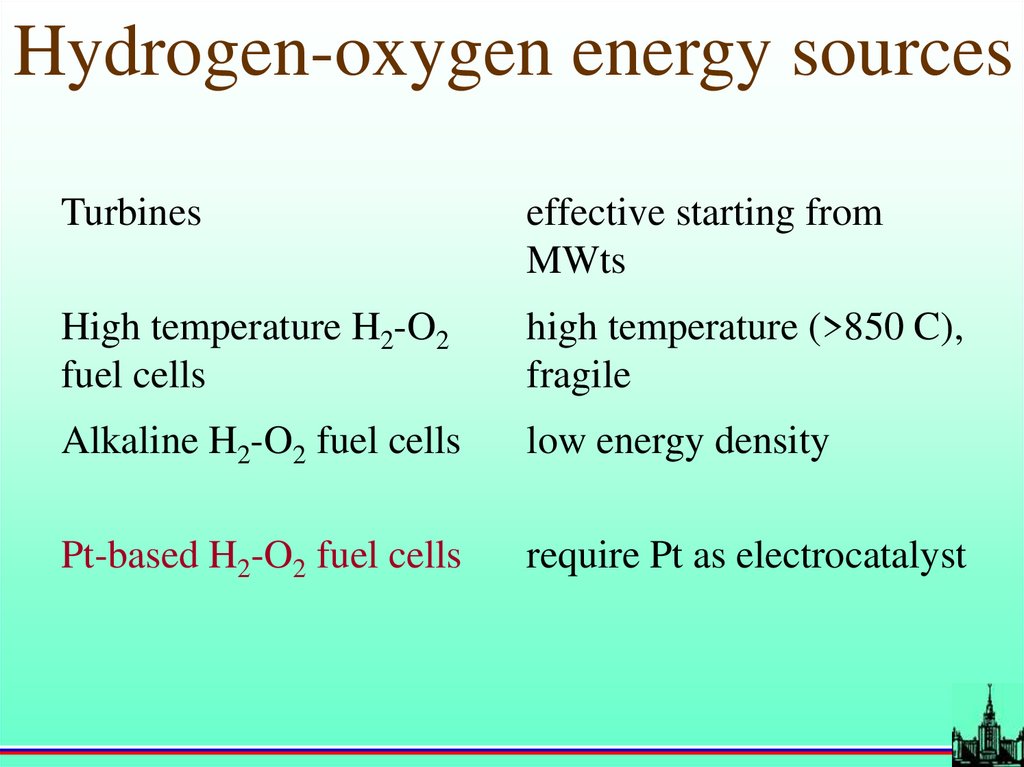
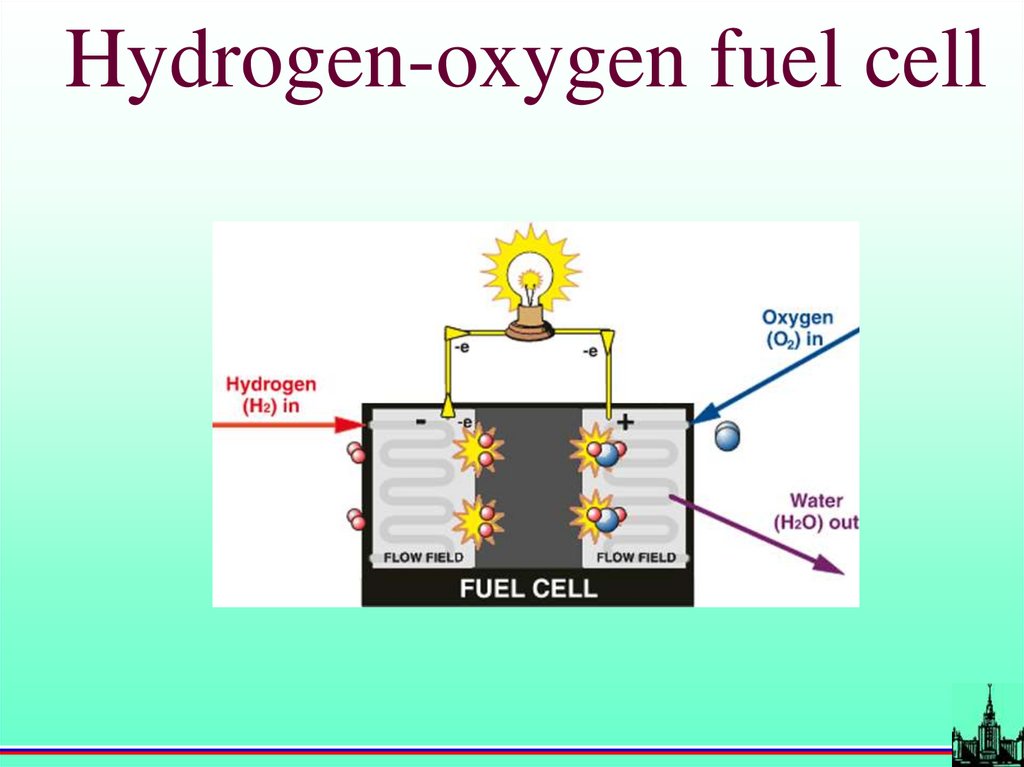
Hydrogen-oxygen energy sourcesTurbines
effective starting from
MWts
High temperature H2-O2
fuel cells
high temperature (>850 C),
fragile
Alkaline H2-O2 fuel cells
low energy density
Pt-based H2-O2 fuel cells
require Pt as electrocatalyst
57.
Hydrogen-oxygen fuel cell58.
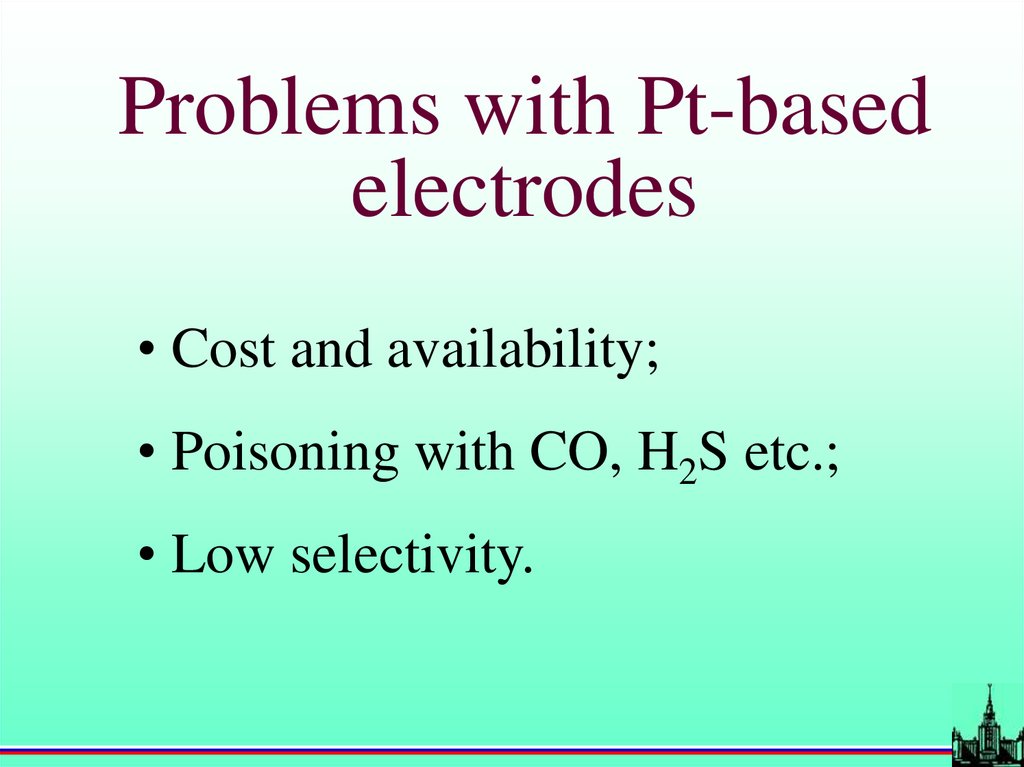
Problems with Pt-basedelectrodes
• Cost and availability;
• Poisoning with CO, H2S etc.;
• Low selectivity.
59.
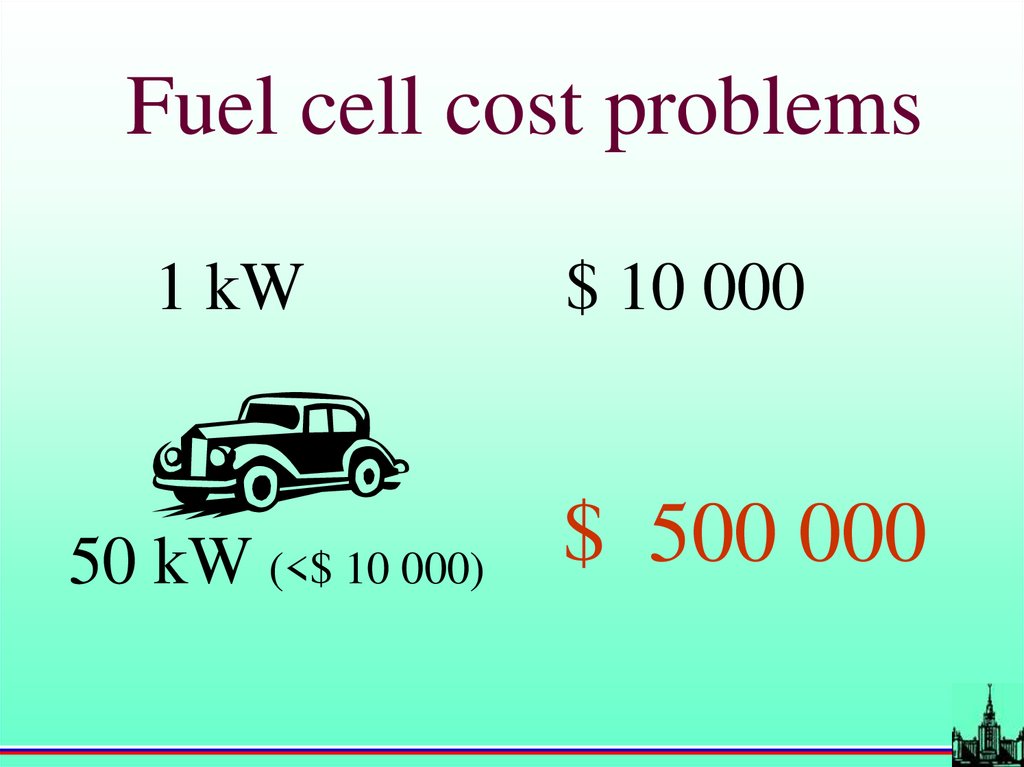
Fuel cell cost problems1 kW
50 kW (<$ 10 000)
$ 10 000
$ 500 000
60.
Dynamics of Pt costPlatinum price / US$ per gramm
70
60
50
40
30
20
10
0
1960
1970
1980
Year
1990
2000
61.
Available amount of PtAnnual production:
Assured resources:
130 tonnes
100 000 tonnes
every year: >60 · 106 cars
2 g of Pt per kW
50 kW engines
> 6 000 tonnes Pt
62.
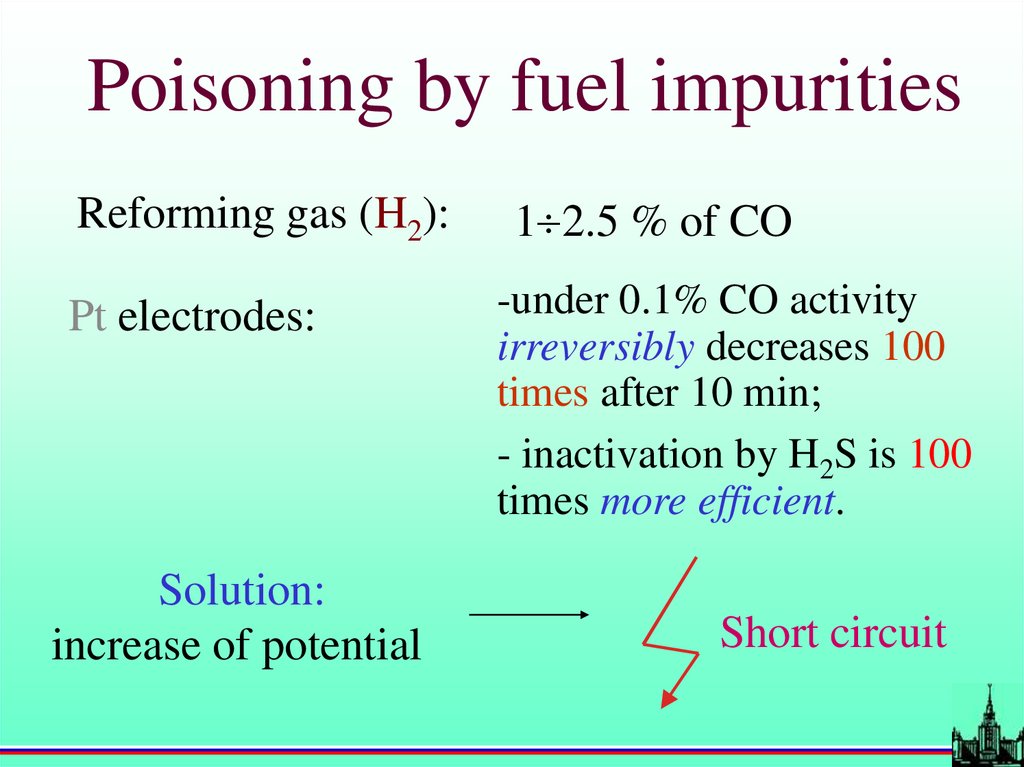
Poisoning by fuel impuritiesReforming gas (H2):
Pt electrodes:
Solution:
increase of potential
1 2.5 % of CO
-under 0.1% CO activity
irreversibly decreases 100
times after 10 min;
- inactivation by H2S is 100
times more efficient.
Short circuit
63.
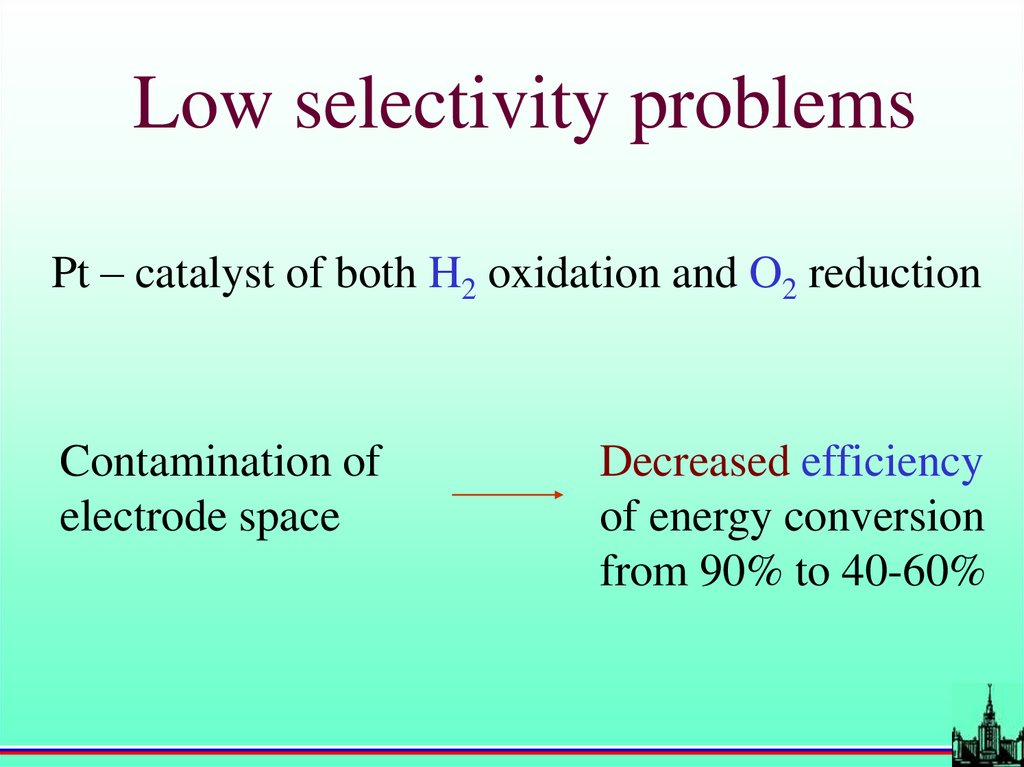
Low selectivity problemsPt – catalyst of both H2 oxidation and O2 reduction
Contamination of
electrode space
Decreased efficiency
of energy conversion
from 90% to 40-60%
64.
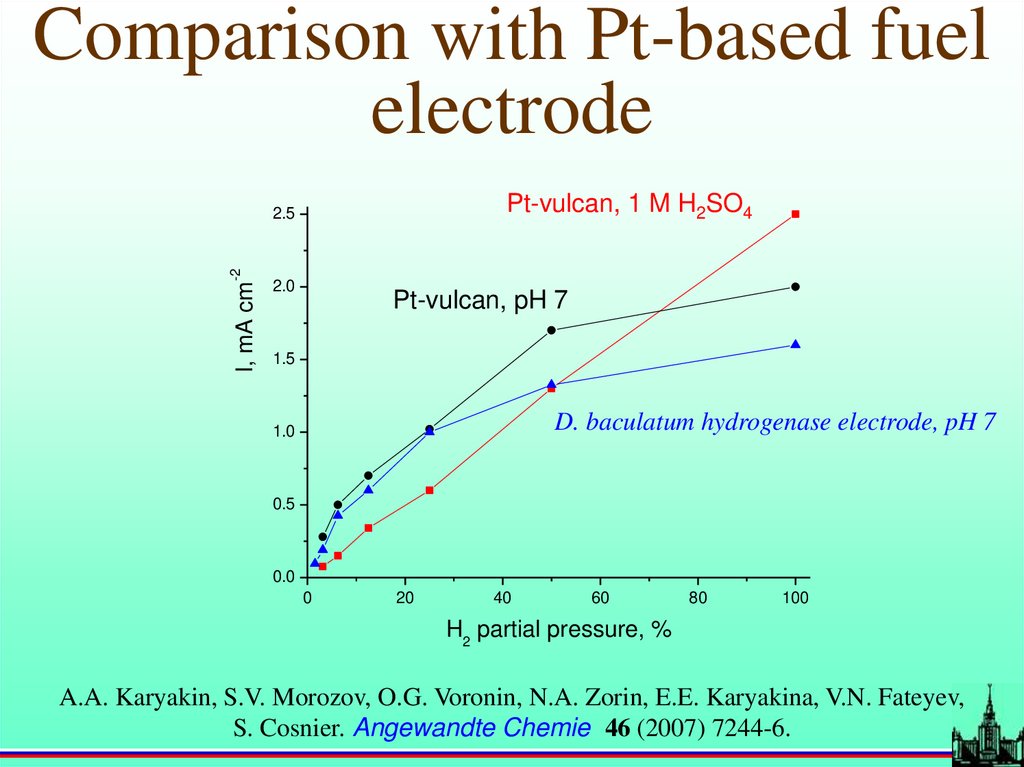
Comparison with Pt-based fuelelectrode
Pt-vulcan, 1 M H2SO4
I, mA cm
-2
2.5
2.0
Pt-vulcan, pH 7
1.5
D. baculatum hydrogenase electrode, pH 7
1.0
0.5
0.0
0
20
40
60
80
100
H2 partial pressure, %
A.A. Karyakin, S.V. Morozov, O.G. Voronin, N.A. Zorin, E.E. Karyakina, V.N. Fateyev,
S. Cosnier. Angewandte Chemie 46 (2007) 7244-6.
65.
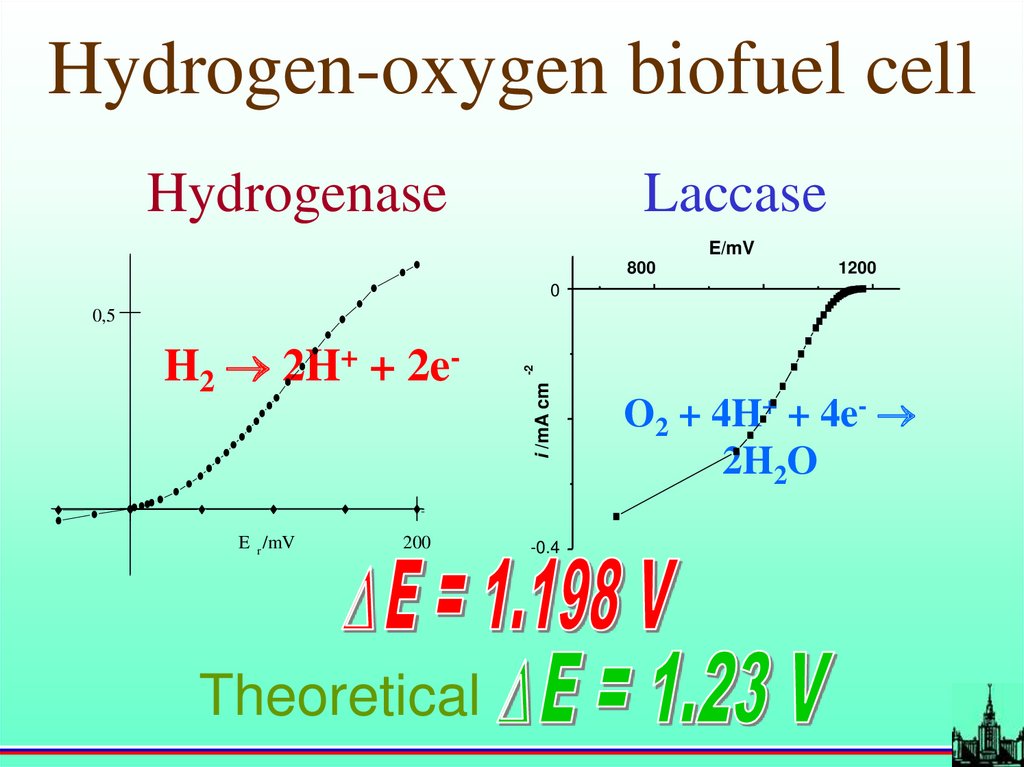
Hydrogen-oxygen biofuel cellHydrogenase
Laccase
E/mV
800
1200
0
E r /mV
200
Theoretical
i /mA cm
H2 2H+ + 2e-
-2
0,5
-0.4
O2 + 4H+ + 4e-
2H2O
66.
Directbioelectrocatalysis by
intact cells
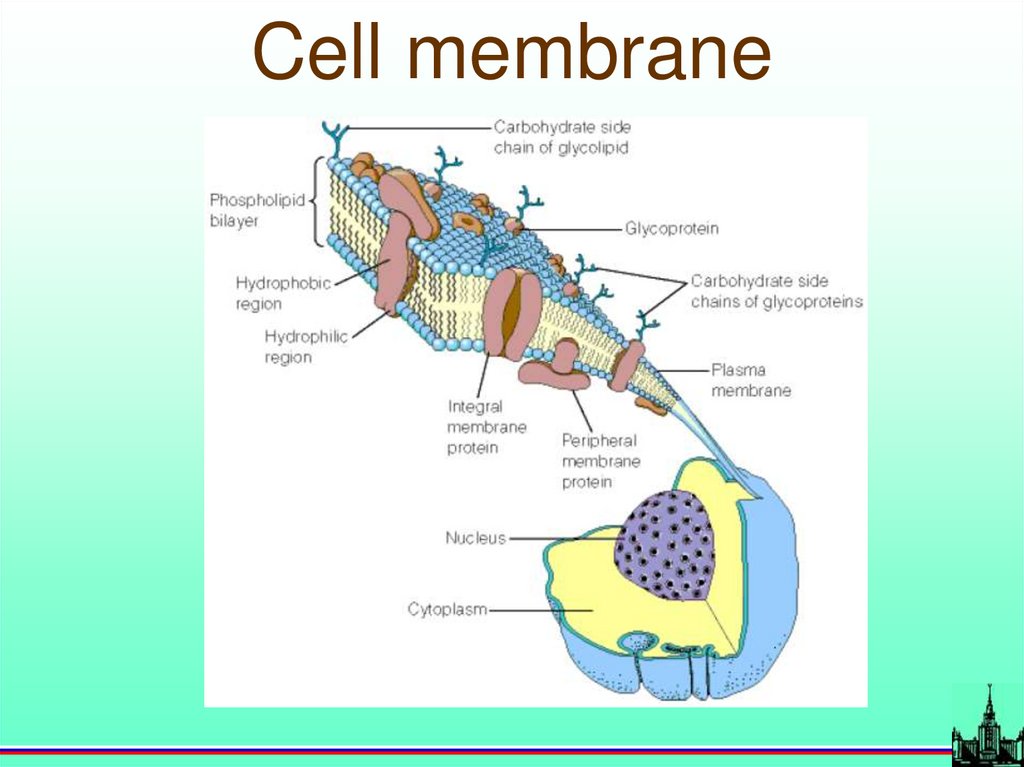
67.
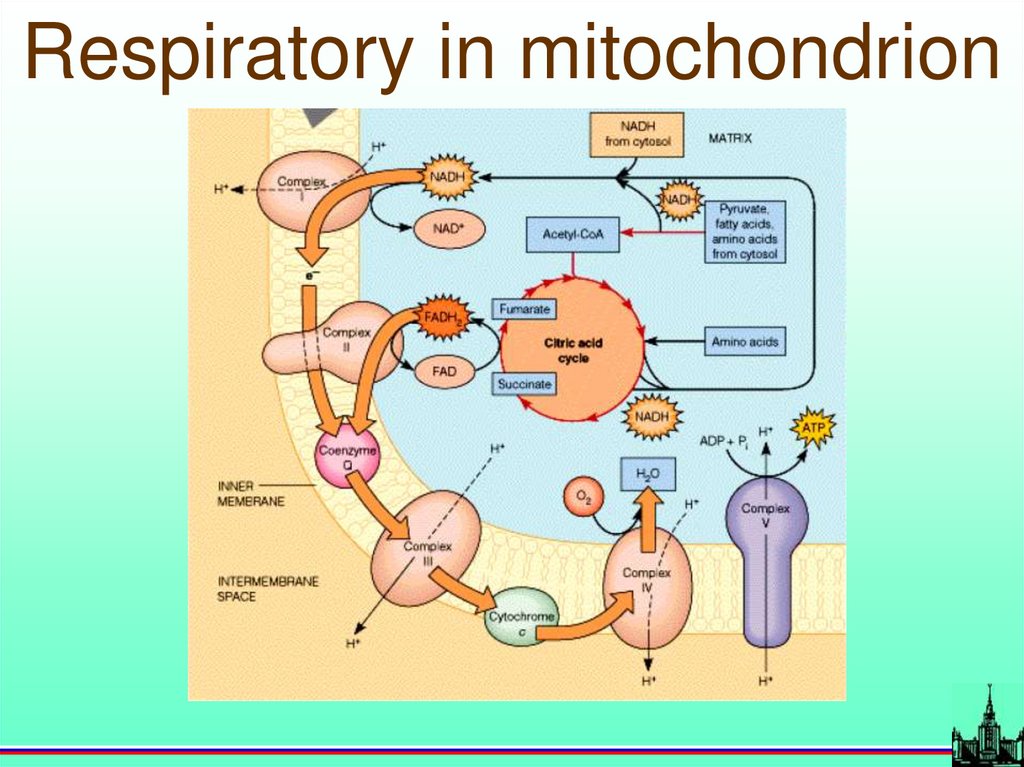
Cell membrane68.
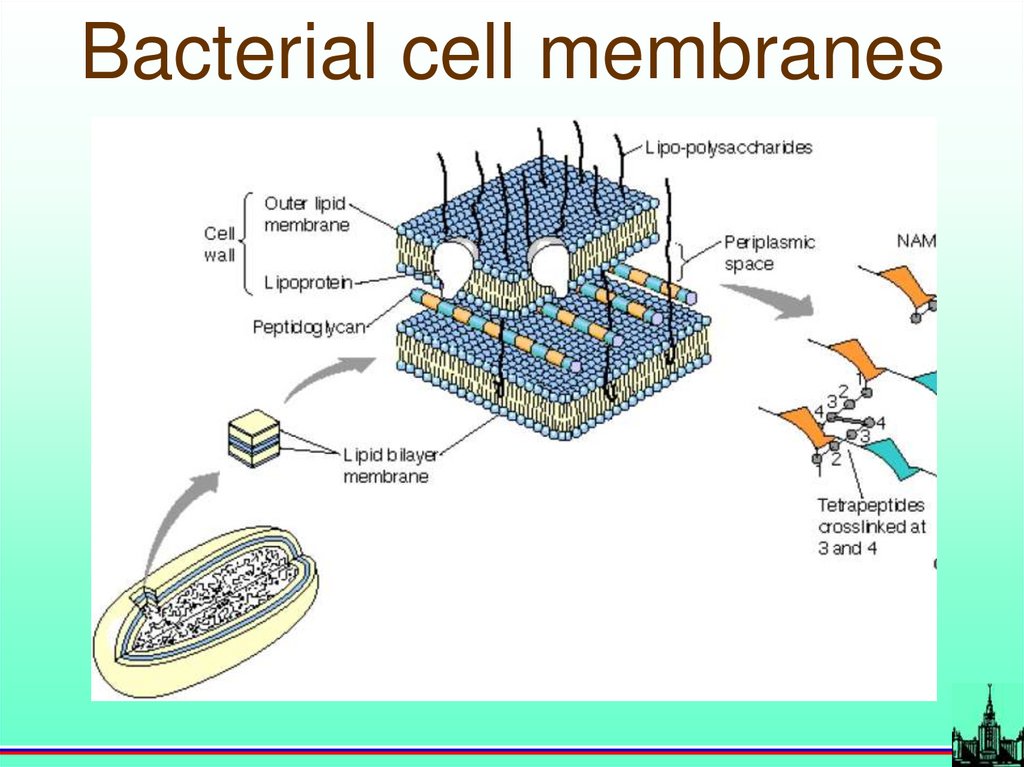
Respiratory in mitochondrion69.
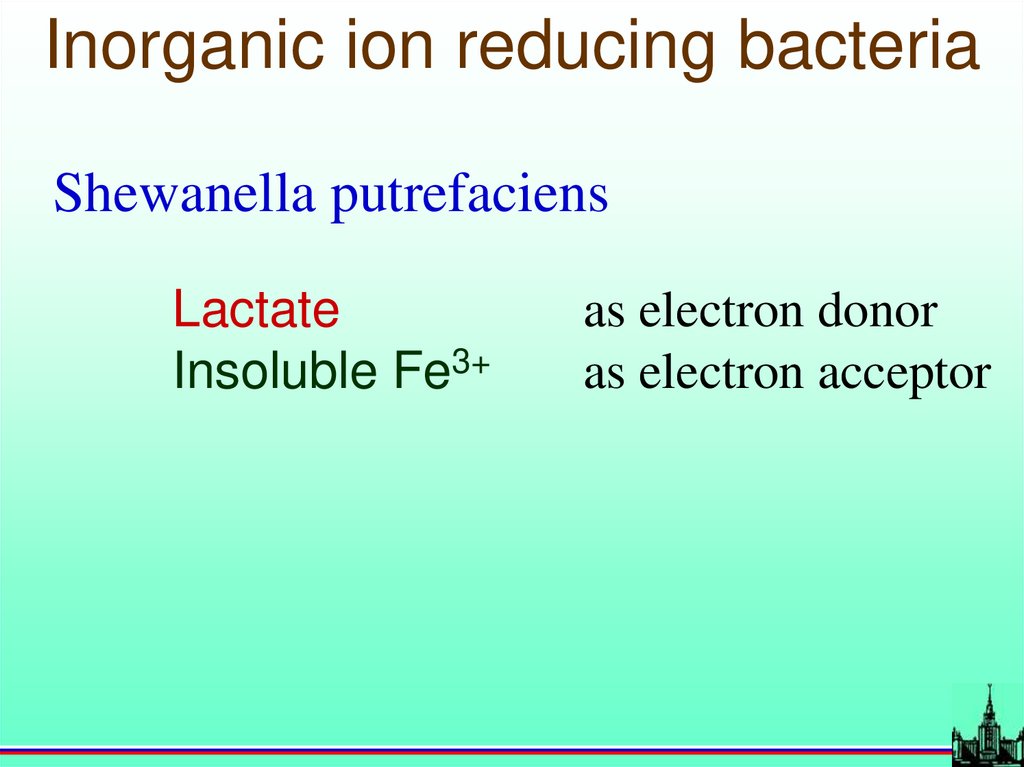
Bacterial cell membranes70.
Inorganic ion reducing bacteriaShewanella putrefaciens
Lactate
Insoluble Fe3+
as electron donor
as electron acceptor
71.
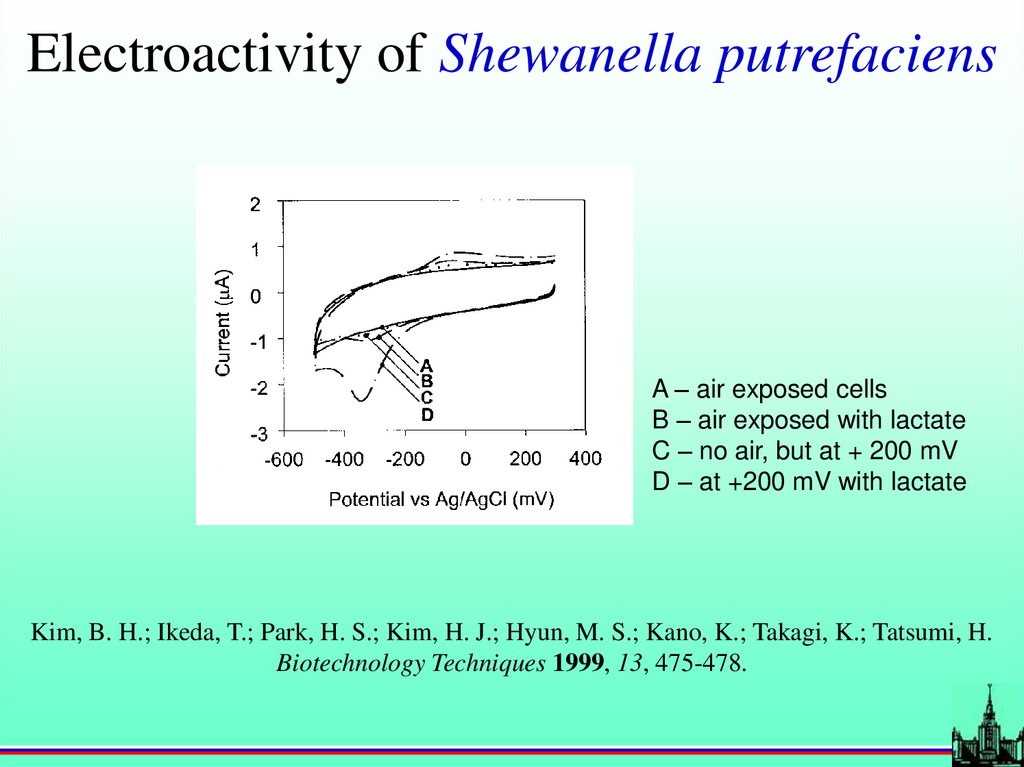
Electroactivity of Shewanella putrefaciensA – air exposed cells
B – air exposed with lactate
C – no air, but at + 200 mV
D – at +200 mV with lactate
Kim, B. H.; Ikeda, T.; Park, H. S.; Kim, H. J.; Hyun, M. S.; Kano, K.; Takagi, K.; Tatsumi, H.
Biotechnology Techniques 1999, 13, 475-478.
72.
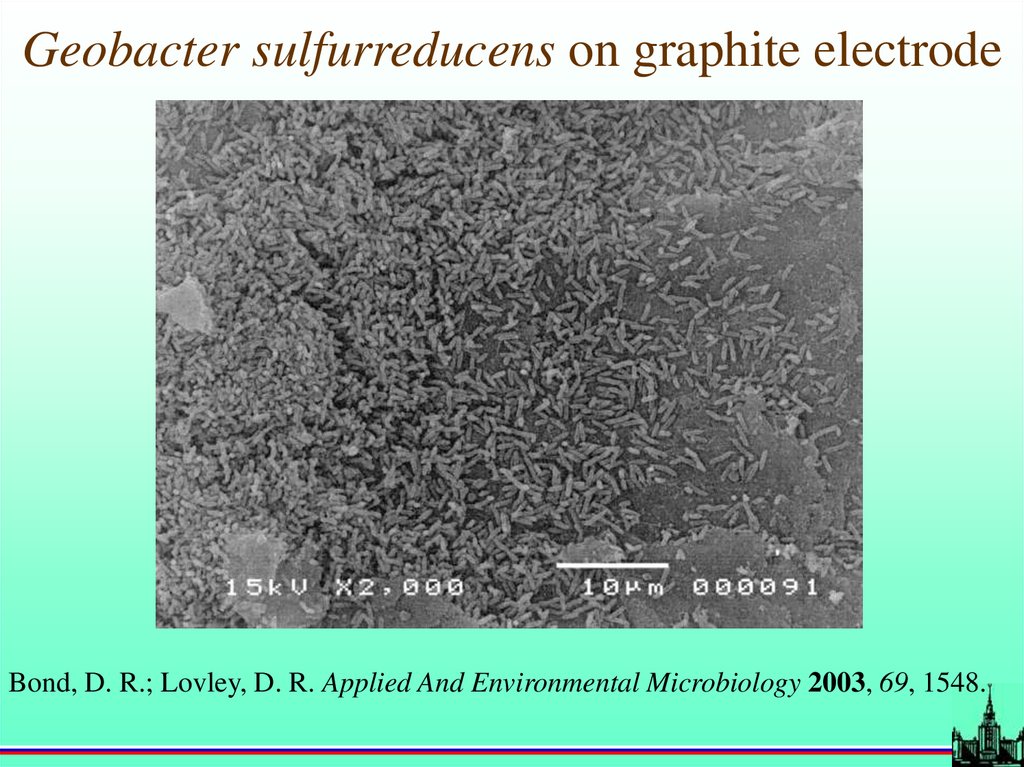
Geobacter sulfurreducens on graphite electrodeBond, D. R.; Lovley, D. R. Applied And Environmental Microbiology 2003, 69, 1548.
73.
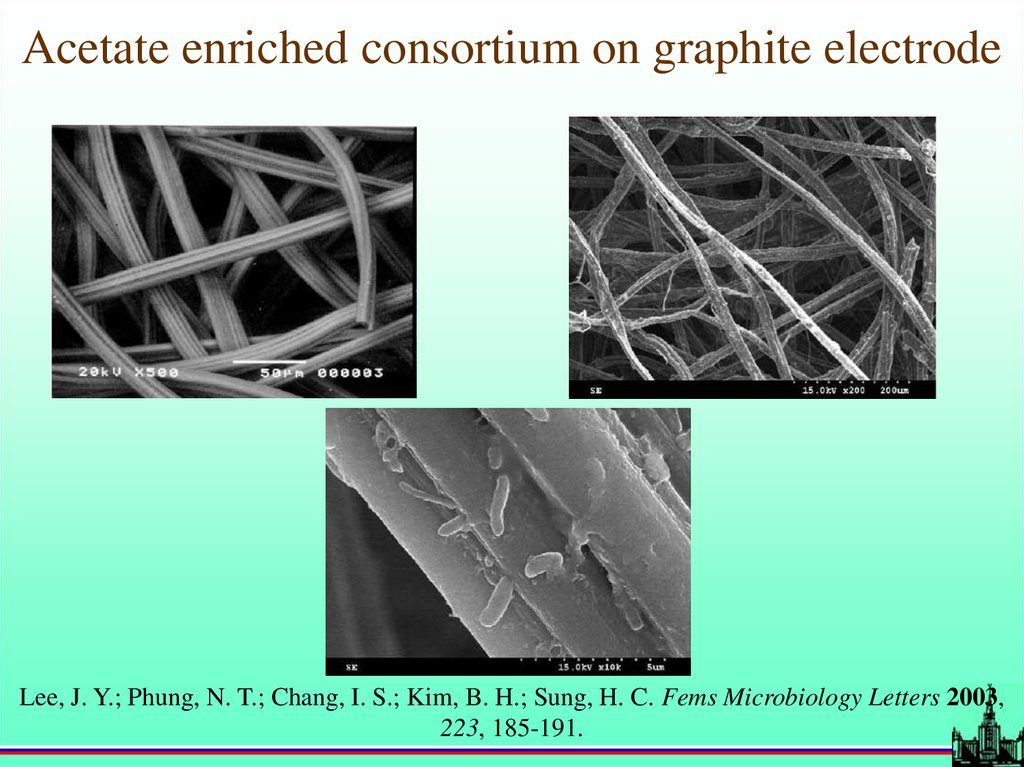
Acetate enriched consortium on graphite electrodeLee, J. Y.; Phung, N. T.; Chang, I. S.; Kim, B. H.; Sung, H. C. Fems Microbiology Letters 2003,
223, 185-191.
74.
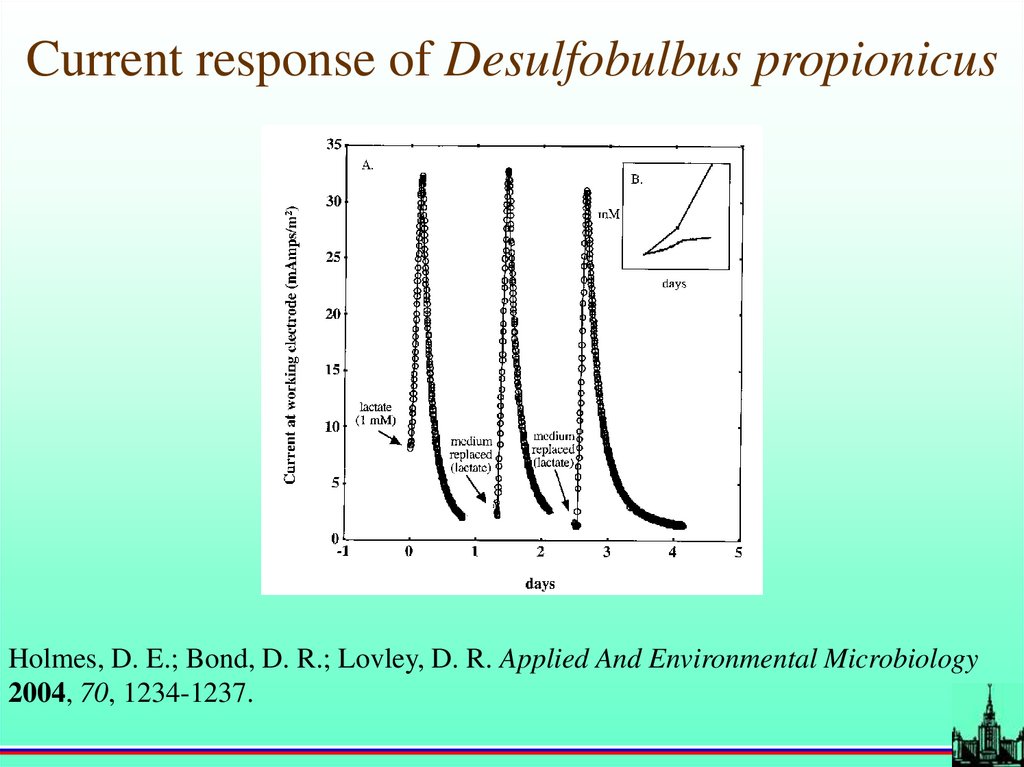
Current response of Desulfobulbus propionicusHolmes, D. E.; Bond, D. R.; Lovley, D. R. Applied And Environmental Microbiology
2004, 70, 1234-1237.
75.
Advantages of bioelectrocatalysis:• a possibility for electrochemistry of complex organic
reactions;
• high efficiency at room temperature and moderate
overvoltages;
• achieve high specificity.
Disadvantages:
• inherent instability,
• large dimensions
of biological catalysts.














 physics
physics chemistry
chemistry








