Similar presentations:
Organic compounds: nomenclature
1.
Organic compounds: nomenclature1
Eight types of systematic nomenclature systems are
recognized by IUPAC. Substitutive and radicofunctional
nomenclatures are the most common.
Example 1: acetone (trivial name).
CH3 C CH3
O
CH3 C CH3
O
propanone
(substitutive name)
dimethyl ketone
(functional class name)
2.
2Trivial names of organic compounds
Formula
Name
Formula
Name
Formula
Name
CH4
methane
C6H14
hexane
CH2=CH2
ethylene
C2H6
ethane
C7H16
heptane
CH3–CH=CH2
propylene
C3H8
propane
C8H18
octane
CH≡CH
acetylene
C4H10
butane
C9H20
nonane
CH2=C–CH=CH2
CH3
isoprene
C5H12
pentane
C10H22
decane
3.
Trivial names of organic compoundsOH
benzene
phenol
C
benzaldehyde
O
C
OH
NH2 aniline
CH3 toluene
O
H
3
benzoic acid
O
furan
NH
pyrrole
S
thiophene
N
pyridine
HCOOH
formic acid
C2H5COOH
propionic acid
CH3COOH
acetic acid
C3H7COOH
butyric acid
4.
Radicofunctional nomenclature4
1. If only one functional group is present in the molecule, then:
list all hydrocarbon radicals in alphabetical order;
add the corresponding class name.
2. If there are two (or more) functional groups in the molecule,
then:
choose the parent structure with a certain trivial name;
list all hydrocarbon radicals and functional groups in
alphabetical order (except those in the parent structure);
add the trivial name of the parent structure;
indicate positions of the substituents using Greek letters
(or prefixes ortho-, meta-, para- for benzene derivatives).
5.
Selected organic compounds and univalent radicals5
6.
Selected organic compounds and univalent radicals6
7.
7Substitutive nomenclature
Systematic nomenclature — a set of terms and rules that
allows to produce a unique name for any substance.
The longest carbon chain that
includes the principal group
or the most complex cyclic
or heterocyclic system
a
b
c
Prefixes
in alphabetical order
etc.
Principal (parent) chain
-ane
-ene
-yne
Suffix indicating
the principal group
Principal group
Suffixes indicating
saturation or unsaturation
of the principal chain
All prefixes and suffixes can be preceded with locants and/or multiplying affixes.
8.
Substitutive nomenclatureSelected Type I substituents in decreasing order of priority:
8
9.
Organic compounds: nomenclatureExample 2: colamine (trivial name).
2
1
NH2 CH2 CH2 OH
NH2 CH2 CH2 OH
2-aminoethanol
(substitutive name)
β-aminoethyl alcohol
(functional class name)
Example 3: medical ether (trivial name).
CH3–CH2–O–CH2–CH3
CH3–CH2–O–CH2–CH3
ethoxyethane
(substitutive name)
diethyl ether
(functional class name)
9
10.
Organic compounds: nomenclature10
Example 4: valine (trivial name).
4
2
1 O
CH3 3
CH CH C
CH3
OH
NH2
2-amino-3-methylbutanoic acid
(substitutive name)
CH3
O
CH CH C
CH3
OH
NH2
α-aminoisopentanoic acid
or
α-aminoisovaleric
acid(functional
class
name)
11.
Organic compounds: nomenclature11
Nomenclature
Substitutive
Radicofunctional
Chemical name
Usually one word
Usually two
or more words
Principal group
Forms suffix
Forms class name
Substituents
Form prefixes
Named separately
Locants
1, 2, 3, ...
(principal group
included)
α, β, γ, ...
(principal group
excluded)
12.
Organic compounds: nomenclatureMore examples:
a)
CH3
CH
CH3
b)
c)
CH3
CH
OH
d) CH2 C CH CH2
e) CH2 CH CH2
OH OH OH
g) CH3
CH3
CH (CH2)4 CH3
CH3
CH3
f)
O
O
C CH2 CH C
HO
OH
NH2
CH3
O
C CH C CH2 C CH C
H
CH2
CH3
Cl
12
13.
13Organic compounds: isomerism
Line formula — a two-dimensional representation of
molecular structure in which atoms are joined by the lines
representing single or multiple bonds, without any indication
of the spatial direction of the bonds.
H
H
C
H
H
H
H
C
H
H
C
H
H
H
C
H
H
H
H
H
Stereochemical formula — a three-dimensional view of
a molecule either as such or in a projection.
14.
Types of isomerism14
principal chain (carbon skeleton)
Constitutional
isomers
Configurational isomers
Conformations*
multiple bond or functional group position
functional group
D- and Lenantiomers
other types
π-diastereomers cis- and transcis- and transdiastereomers
σ-diastereomers more than one
chiral atom
eclipsed
gauche- (skew-)
staggered
anti- (trans-)
*) Usually undergo fast interconversions and are not considered as isomers
15.
15Organic compounds: isomerism
Isomers — molecular entities that have the same atomic
composition (molecular formula) but different line formulas or
different stereochemical formulas and hence different
physical and/or chemical properties.
CH3 CH2 CH2 CH3
CH3 CH2 CH2 CH2 OH
CH3 CH CH3
CH3
CH3 CH2 CH CH3
OH
H3C
CH3
C C
H
H
H3C
H
C C
H
CH3
CH3
C
C2H5
CH3
OH
H
C
HO
H
C2H5
16.
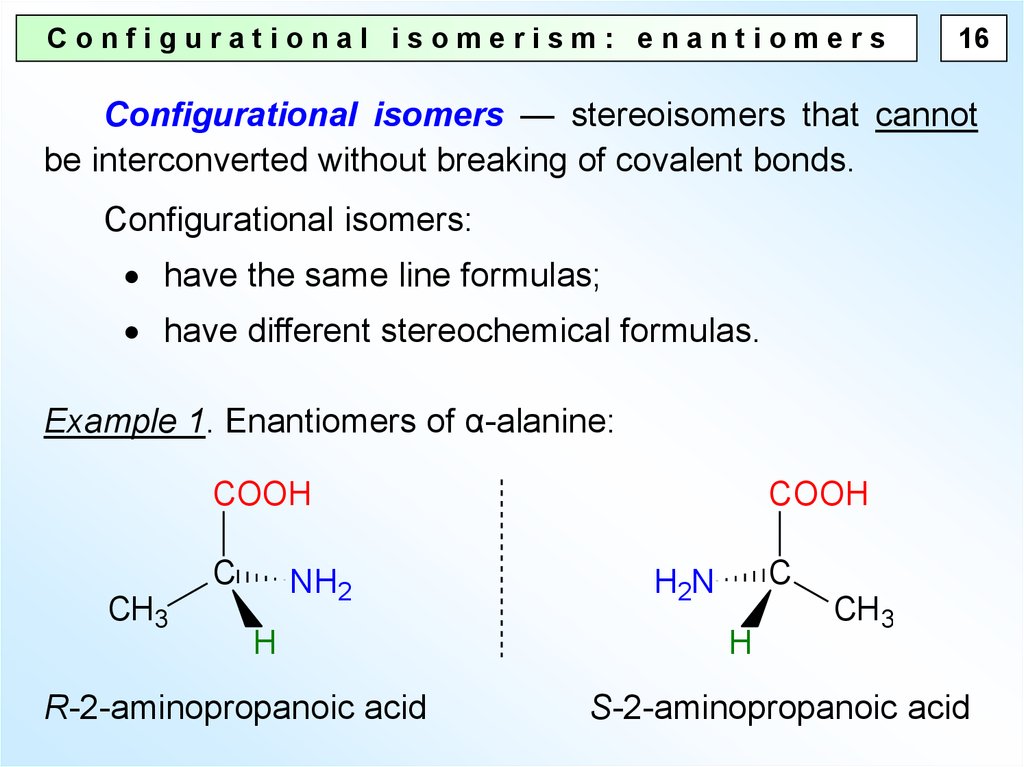
Configurational isomerism: enantiomers16
Configurational isomers — stereoisomers that cannot
be interconverted without breaking of covalent bonds.
Configurational isomers:
have the same line formulas;
have different stereochemical formulas.
Example 1. Enantiomers of α-alanine:
COOH
C
CH3
NH2
H
R-2-aminopropanoic acid
COOH
C
H2N
H
CH3
S-2-aminopropanoic acid
17.
17Fischer projections
COOH
C
CH3
NH2
COOH
H
C
CH3
H
COOH
H
C
H2N
NH2
COOH
H2N
C
H
CH3
CH3
COOH
COOH
H
NH2
CH3
D-alanine
H2N
H
CH3
L-alanine
18.
Electron structure of ethyleneSpatial configuration of σ- and π-bonds:
18
19.
19Configurational isomerism: diastereomers
Example 2. Two π-diastereomers of 9-octadecenoic acid:
H
H
C
CH3(CH2)7
CH3(CH2)7
H
C
C
(CH2)7COOH
oleic acid
(cis-9-octadecenoic acid)
liquid (b. p. 13–16 °C)
Z-isomer
H
C
(CH2)7COOH
elaidic acid
(trans-9-octadecenoic acid)
solid (b. p. 44–47 °C)
E-isomer
20.
20Configurational isomerism: diastereomers
Example 3. Enantiomers and σ-diastereomers of 1,2-cyclohexanediol:
OH
OH
trans-
OH
OH OH
cis-
OH
trans-
21.
21Configurational isomerism: diastereomers
Example 4. Enantiomers and σ-diastereomers of 1,3-cyclohexanediol:
OH
HO
HO
OH
HO
trans-
cis-
OH
trans-
22.
22Conformational isomerism
Conformational isomers — stereoisomers that can be
interconverted without breaking of covalent bonds.
Example 5. Conformations of ethane:
H
H
C
H
H
H
C
C
H
H
H
H
H
H
H
HH
H
H
C
H
H
H
Eclipsed
H
H
H
H
H
Staggered
23.
23Conformational isomerism
HH
H
H
H
H
H
Eclipsed
Torsion (eclipsional) strain: 12 kJ/mol
H
H
H
H
H
Staggered
24.
24Conformational isomerism
Example 6. Conformations of ethyleneglycol:
HOOH
H
H
H
H
OH
H
OH
H
H
H
Eclipsed – 1 (syn)
Staggered – 1 (gauche)
HOH
OH
H
H
H
OH
Eclipsed – 2
H
H
H
H
OH
Staggered – 2 (anti)























 chemistry
chemistry








