Similar presentations:
You must match the right definition or word in your bingo board if you finish all say Bingo
1.
Your teacher will show an slidewith a definition or a word
You must match the right definition
or word in your bingo board
If you finish all say BINGO
Write the answer in your Carton
2.
1. Ammonia3.
2. Ammonium4.
3. ammonium hydroxidesolution
NH3(g) + H20(l) --> NH4OH(aq)
5.
4. Ammoniumchloride heated
6.
5. Products of reactioncan themselves react
to produce the original
react
7.
6. Forward reactionneeds heat to form the
products . Delta H +
8.
7. Where nothing can getin/out
• Appartus for a reaction where
no substances
9.
8. 1) conc2) temp
3) pressure
10.
• Know and understand theenvironmental impact of oxides of
nitrogen in the atmosphere and
nitrates in soils and water supplies.
• Know and understand the chemistry
of ammonia as a gas and in aqueous
solution.
11. Why we study ammonia?
12. What is ammonia?
Ammonia is an important compoundin the manufacture of fertilizer and
other chemicals such as cleaning
fluids and floor waxes.
It is made industrially by reacting
nitrogen with hydrogen in the Haber
process. It is a reversible reaction,
so it never goes to completion.
Why is this a problem for companies
making ammonia?
nitrogen
+
hydrogen
ammonia
N2 (g)
+
3H2 (g)
2NH3 (g)
13. Ammonia is an important industrial chemical.
- In groups use a pcto investigate.
- You must present
a small PPT about
one use of
ammonia.
- Relevant things
production.
countries, export
etc.
- Water impact
14.
AmmoniaAwareness
14
15.
Properties of Ammonia (NH3)Ammonia gas is:
the most water
soluble
of all gases
a colorless gas with a
very pungent odor
lighter than air
Ammonia liquid is:
lighter than water
15
16.
AmmoniaDetection
The nose is sensitive to
the presence of
ammonia gas in the air
because of its very
pungent odor
Ammonia in the air
appears as a dense
heavy fog
16
17. Ammonia Hazards
• Caustic (alkali) burns,•Acute ammonia acts corrosively to bare skin
• Freezing burns
• Inhalation danger to
lining of mouth, throat
and lungs
• Does not support
respiration – suffocation
danger
• Especially dangerous to
eyes
18. Response
Water, water, water!Carry a squirt bottle on
your person and in your
vehicle for immediate
first aid to eyes.
Have a water hose installed
near all storage tanks.
Know the location of
showers and eye wash
stations.
19.
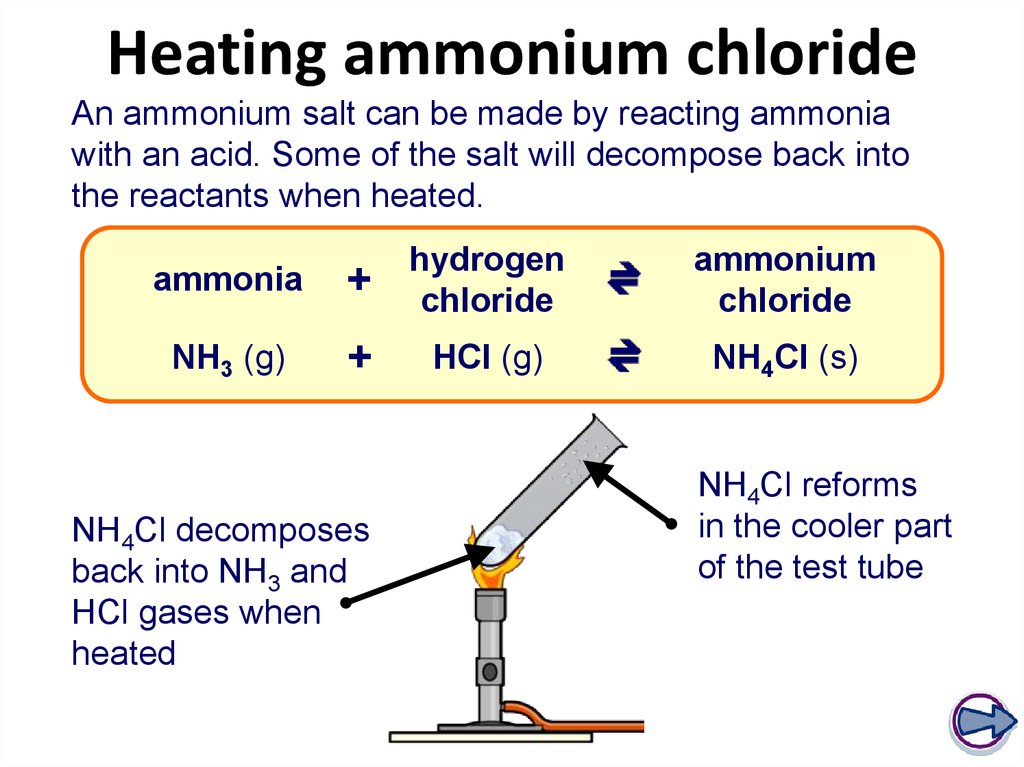
20. Heating ammonium chloride
An ammonium salt can be made by reacting ammoniawith an acid. Some of the salt will decompose back into
the reactants when heated.
ammonia
+
hydrogen
chloride
NH3 (g)
+
HCl (g)
NH4Cl decomposes
back into NH3 and
HCl gases when
heated
ammonium
chloride
NH4Cl (s)
NH4Cl reforms
in the cooler part
of the test tube
21.
EQUILIBRIUM REACTIONSInitially, there is no backward reaction but, as products form, it speeds up and
provided the temperature remains constant there will come a time when the backward
and forward reactions are equal and opposite; the reaction has reached equilibrium.
FASTEST AT THE START
NO BACKWARD REACTION
FORWARD REACTION SLOWS DOWN
AS REACTANTS ARE USED UP
BACKWARD REACTION
STARTS TO INCREASE
In an equilibrium reaction, not all the
reactants end up as products; there is not a
100% conversion.
BUT IT DOESN’T MEAN THE REACTION
IS STUCK IN THE MIDDLE
AT EQUILIBRIUM THE BACKWARD
AND FORWARD REACTIONS ARE
EQUAL AND OPPOSITE
22.
23. Reversible or irreversible?
24.
25. The chemical reaction that feeds the world
https://www.youtube.com/watch?v=o1_D4FscMnU26. Anagrams
27. The Haber process
28. What is yield?
The amount of product made in a reaction is called theyield and is usually expressed as a percentage.
ammonia yield (%)
The yield of ammonia produced by the Haber process
depends on the temperature and pressure of the reaction.
pressure (atm)
29. What is the Haber compromise?
The highest yield of ammoniais theoretically produced by
using a low temperature and
a high pressure.
In practice, though, these
conditions are not used. Why?
Lowering the temperature slows down the rate of reaction.
This means it takes longer for ammonia to be produced.
Increasing the pressure means stronger, more expensive
equipment is needed. This increases the cost of producing
the ammonia.
A compromise is reached to make an acceptable yield in
a reasonable timeframe while keeping costs down.
30. Temperature, pressure and yield
31. Changing the yield of ammonia
32.
HABER PROCESSConditions
N2(g) + 3H2(g)
Pressure
Temperature
Catalyst
2NH3(g)
: DH = - 92 kJ mol-1
20000 kPa (200 atmospheres)
380-450°C
iron
33.
HABER PROCESSN2(g) + 3H2(g)
2NH3(g)
: DH = - 92 kJ mol-1
Conditions
Pressure
20000 kPa (200 atmospheres)
Temperature
380-450°C
Catalyst
iron
Equilibrium theory favours
low temperature
exothermic reaction - higher yield at lower temperature
high pressure
decrease in number of gaseous molecules
34.
HABER PROCESSN2(g) + 3H2(g)
2NH3(g)
: DH = - 92 kJ mol-1
Conditions
Pressure
20000 kPa (200 atmospheres)
Temperature
380-450°C
Catalyst
iron
Equilibrium theory favours
low temperature
exothermic reaction - higher yield at lower temperature
high pressure
decrease in number of gaseous molecules
Kinetic theory favours
high temperature
greater average energy + more frequent collisions
high pressure
more frequent collisions for gaseous molecules
catalyst
lower activation energy
35.
HABER PROCESSN2(g) + 3H2(g)
2NH3(g)
: DH = - 92 kJ mol-1
Conditions
Pressure
20000 kPa (200 atmospheres)
Temperature
380-450°C
Catalyst
iron
Equilibrium theory favours
low temperature
exothermic reaction - higher yield at lower temperature
high pressure
decrease in number of gaseous molecules
Kinetic theory favours
high temperature
greater average energy + more frequent collisions
high pressure
more frequent collisions for gaseous molecules
catalyst
lower activation energy
Compromise conditions
Which is better?
A low yield in a shorter time
or
a high yield over a longer period.
The conditions used are a compromise with the catalyst
enabling the rate to be kept up, even at a lower temperature.
36.
HABER PROCESSIMPORTANT USES OF AMMONIA AND ITS COMPOUNDS
MAKING
FERTILISERS
80% of the ammonia produced goes to make fertilisers such as
ammonium nitrate (NITRAM) and ammonium sulphate
NH3 + HNO3
——>
2NH3 + H2SO4 ——>
MAKING
NITRIC ACID
NH4NO3
(NH4)2SO4
ammonia can be oxidised to nitric acid
nitric acid is used to manufacture...
fertilisers (ammonium nitrate)
explosives (TNT)
polyamide polymers (NYLON)
37. The Haber compromise
To produce a high yield of ammonia, but with a fast rateof reaction and without the need for overly expensive
equipment, the Haber process is carried out at 450 °C
and 200 atmospheres.
The most important factor in
deciding what conditions to use is
therefore not yield, but total cost.
What costs are involved in
the industrial production of
ammonia?
raw materials
equipment
energy
wages
38. Maximizing productivity
What else can be done to maximise productivity in themanufacture of ammonia?
An iron catalyst is used to increase the rate of
reaction. It speeds up both the forward and backward
reaction, so the position of equilibrium is not affected.
The ammonia is cooled, liquefied and then removed
as it is produced. This causes the equilibrium to shift to
the right to produce more ammonia.
Unreacted nitrogen and hydrogen are recycled and
given another chance to react.
39. Demonstration LAB
Pay attention to what your teacher will showyou , takes notes about colour change during
the reaction.
Fe, Cu, Zn, conc. HNO3
40.
Created your Haber process
Show in a creative map the process
Main stages
And a sketch of the plant
• Students will glue posters around and
other go and check other ideas.
41.
Pair workOrganize the stages of the Haber process











































 chemistry
chemistry