Similar presentations:
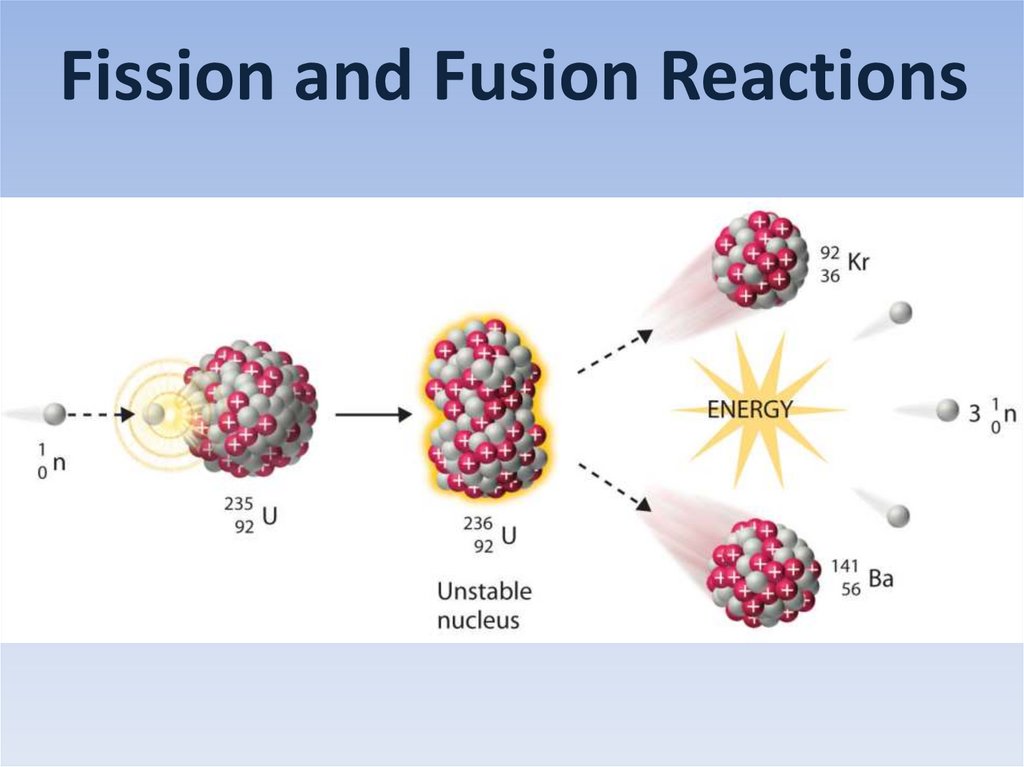
Fission and Fusion Reactions
1.
Fission and Fusion Reactions2.
Topics/ Learning ObjectivesNuclear reactions Vs. chemical reactions
Nuclear fission
Nuclear fusion
MCA
3.
Nuclear ReactionsMCA
Two main types of nuclear reactions
• Fission: A heavy unstable nucleus splits into two more stable isotopes
• Fusion: Two light nuclei are combined to form a heavier nucleus
Both reactions release great amounts of energy
Nuclear fission
(induced fission)
Ba-139
Kr-95
Nuclear fusion
4.
Nuclear FusionMCA
In a fusion nuclear reaction, two light nuclei join to form a heavier
element
Example
2
1
H H
3
1
He n energy
4
2
1
0
5.
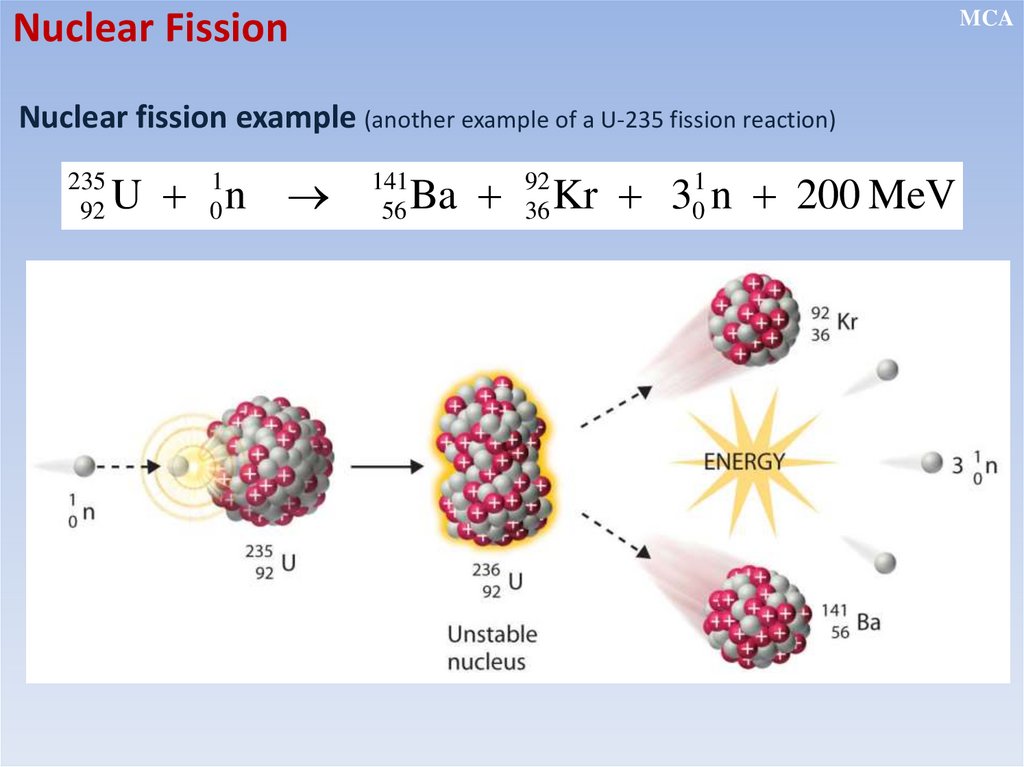
Nuclear FissionMCA
Nuclear fission example (another example of a U-235 fission reaction)
235
92
U 01n
141
56
1
Ba 92
Kr
3
36
0 n 200 MeV
6.
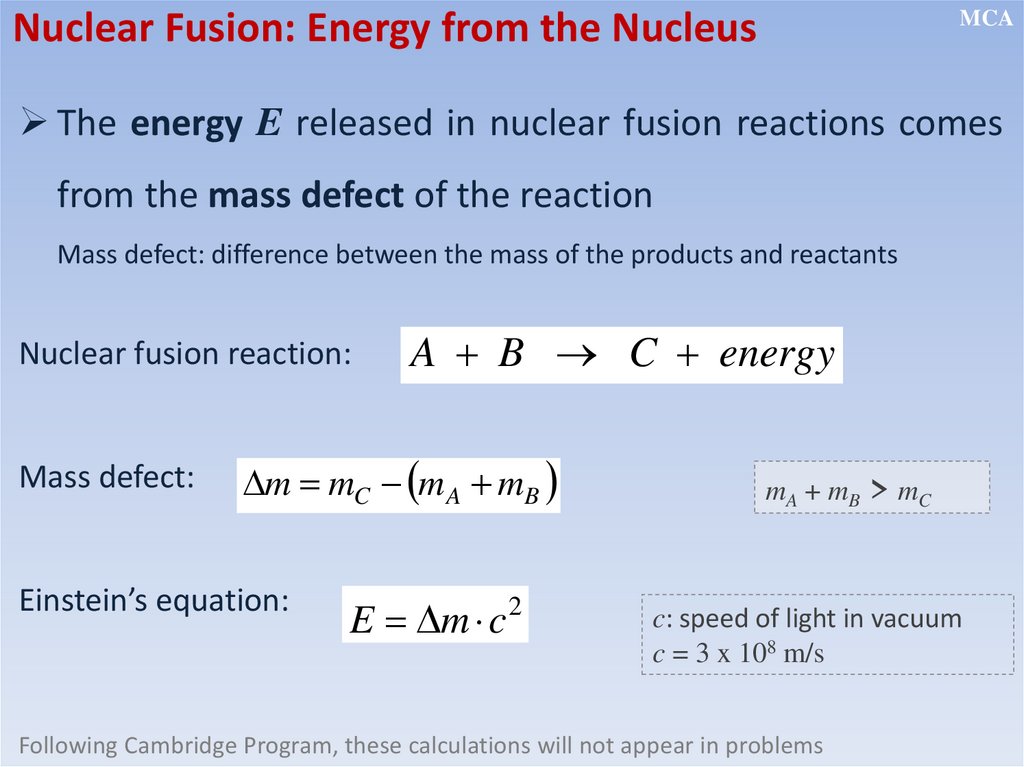
Nuclear Fusion: Energy from the NucleusMCA
The energy E released in nuclear fusion reactions comes
from the mass defect of the reaction
Mass defect: difference between the mass of the products and reactants
Nuclear fusion reaction:
Mass defect:
A B C energy
m mC m A mB
Einstein’s equation:
E m c
2
mA + mB > mC
c: speed of light in vacuum
c = 3 x 108 m/s
Following Cambridge Program, these calculations will not appear in problems
7.
Nuclear Fission: Energy from the NucleusMCA
The energy E released in nuclear fission reactions comes
from the mass defect of the reaction
Mass defect: difference between the mass of the products and reactants
Nuclear fission reaction:
Mass defect:
X Y Z energy
Δm mY mZ m X
Einstein’s equation:
E m c
2
mX > m Y + mZ
c: speed of light in vacuum
c = 3 x 108 m/s
Following Cambridge Program, these calculations will not appear in problems
8.
9.
1. 1 unit mass = 1,66 ∗ 10−27 kg−19
2. 1 eV = 1,6 ∗ 10 J
3. 1 MeV= 1 ∗ 106 eV = 1,6 ∗ 10−13 J






 chemistry
chemistry








