Similar presentations:
Colloid chemistry
1.
Zaporozhye statemedical University
Department of physical and
colloid chemistry
COLLOID
CHEMISTRY
1
2.
Signs of colloid chemistry objects1. Heterogeneity (multiphase).
2. Dispersion (fragmentation).
Colloidal chemistry is sometimes called
PHYSICOCHEMISTRY disperse systems
2
3.
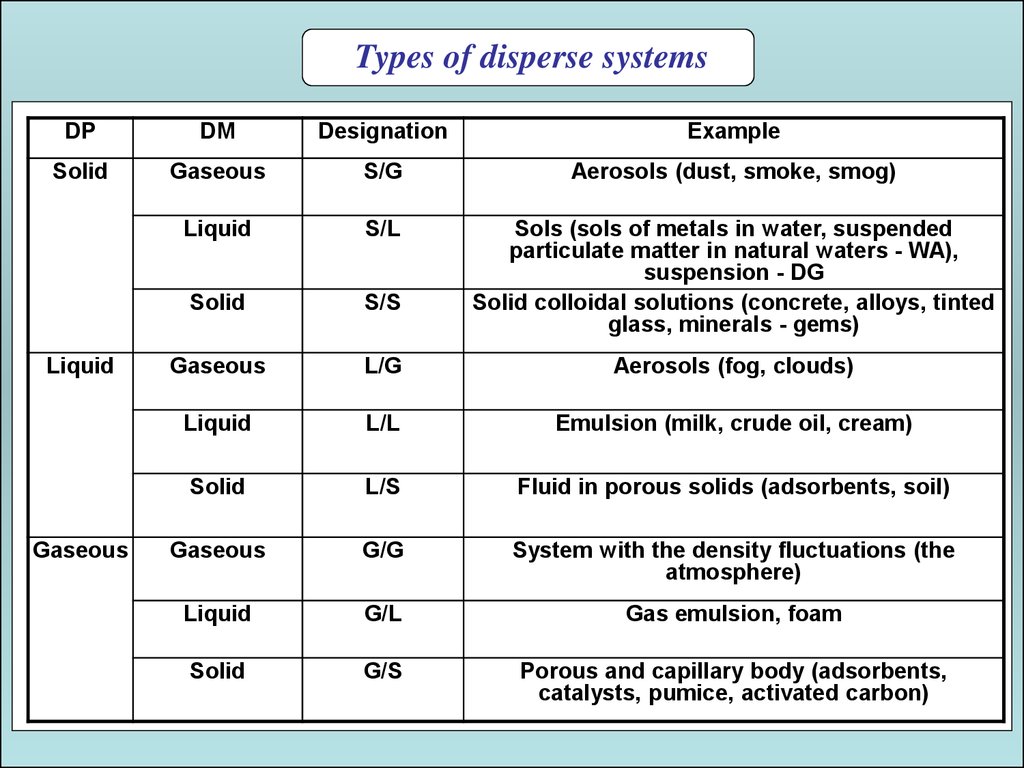
Types of disperse systemsDP
DM
Designation
Example
Solid
Gaseous
S/G
Aerosols (dust, smoke, smog)
Liquid
S/L
Solid
S/S
Sols (sols of metals in water, suspended
particulate matter in natural waters - WA),
suspension - DG
Solid colloidal solutions (concrete, alloys, tinted
glass, minerals - gems)
Gaseous
L/G
Aerosols (fog, clouds)
Liquid
L/L
Emulsion (milk, crude oil, cream)
Solid
L/S
Fluid in porous solids (adsorbents, soil)
Gaseous
G/G
System with the density fluctuations (the
atmosphere)
Liquid
G/L
Gas emulsion, foam
Solid
G/S
Porous and capillary body (adsorbents,
catalysts, pumice, activated carbon)
Liquid
Gaseous
3
4.
Classification by degree of interactiondispersion phase with the dispersion medium
Lyophilic - a systems, where interaction of the particles of
the dispersed phase with the solvent is highly expressed.
Lyophobic - dispersion phase interacts weakly with the
dispersion medium.
Hydrophilic (a) and
hydrophobic (b) surface in a
three phase system - water solid - air; 1 - Water 2; - Solid;
3 - air; a - wetting angle.
4
5.
Features of colloidal systems1. Excess surface energy GS
GS S
2. Thermodynamic instability
3. Irreproducibility
4. Capacity to structure formation
5
6.
Obtaining disperse systemsDispersion methods
-grinding large sample substance to
disperse particles sizes;
6
7.
Colloid millsAllow to reach more subtle grinding
7
8.
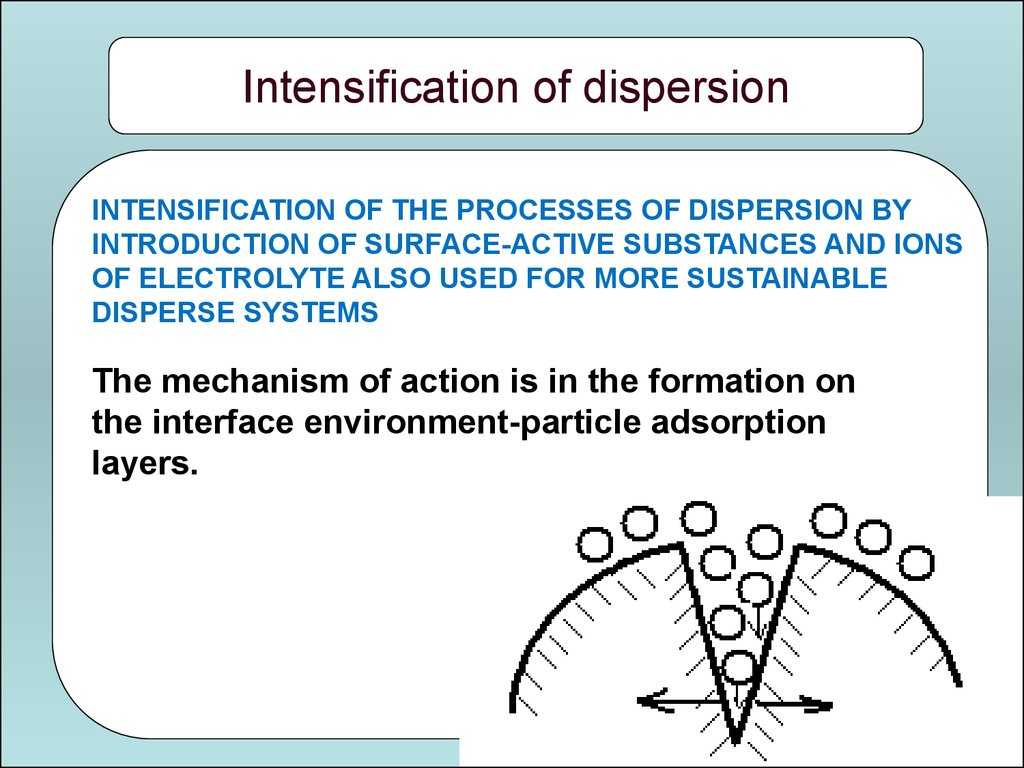
Intensification of dispersionINTENSIFICATION OF THE PROCESSES OF DISPERSION BY
INTRODUCTION OF SURFACE-ACTIVE SUBSTANCES AND IONS
OF ELECTROLYTE ALSO USED FOR MORE SUSTAINABLE
DISPERSE SYSTEMS
The mechanism of action is in the formation on
the interface environment-particle adsorption
layers.
8
9.
Condensation methods• based on the association of molecules in aggregates from
true solutions;
• used to obtain highly dispersed systems;
• do not require the cost of external work;
• emergence of a new phase occurs at a supersaturation
environment.
9
10.
Condensation stage1. Germ-formation - the emergence of centers of
crystallization.
2. Growth of the germ.
3. Formation of a layer of stabilizer (DEL).
10
11.
Physical condensation methods1. Method of condensation of vapor - the formation
of mist in the gas phase at low temperatures.
2. Method of replacing the solvent - is poured into a
solution of a liquid substance in which the substance is
substantially insoluble.
11
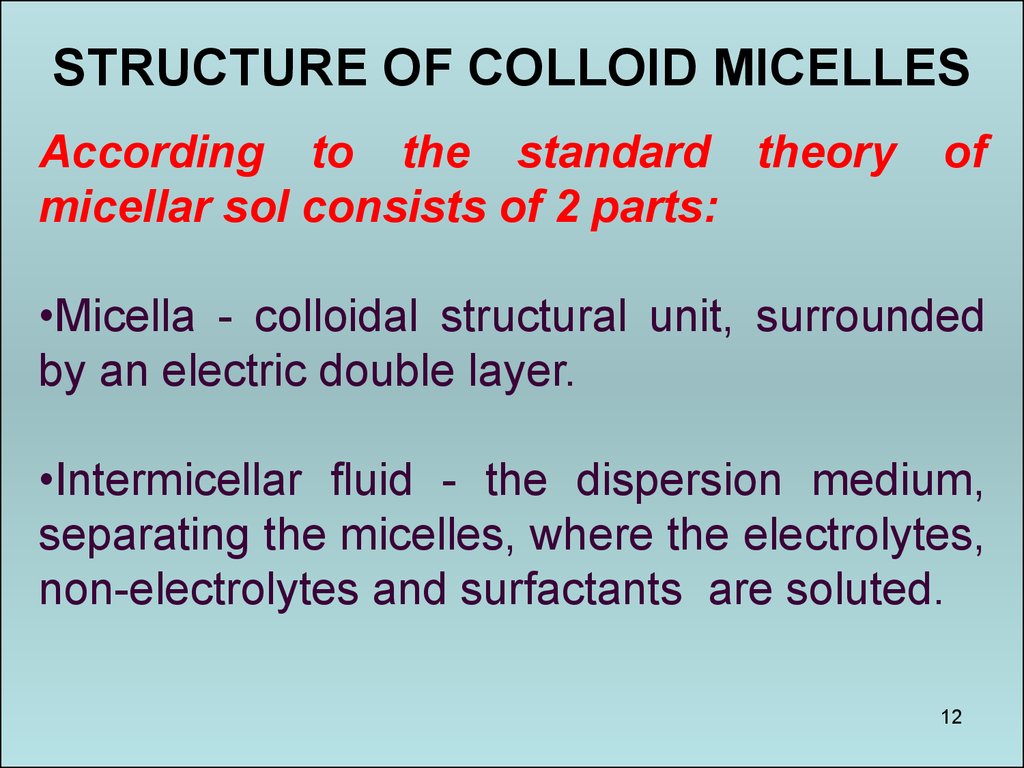
12.
STRUCTURE OF COLLOID MICELLESAccording to the standard theory
micellar sol consists of 2 parts:
of
•Micella - colloidal structural unit, surrounded
by an electric double layer.
•Intermicellar fluid - the dispersion medium,
separating the micelles, where the electrolytes,
non-electrolytes and surfactants are soluted.
12
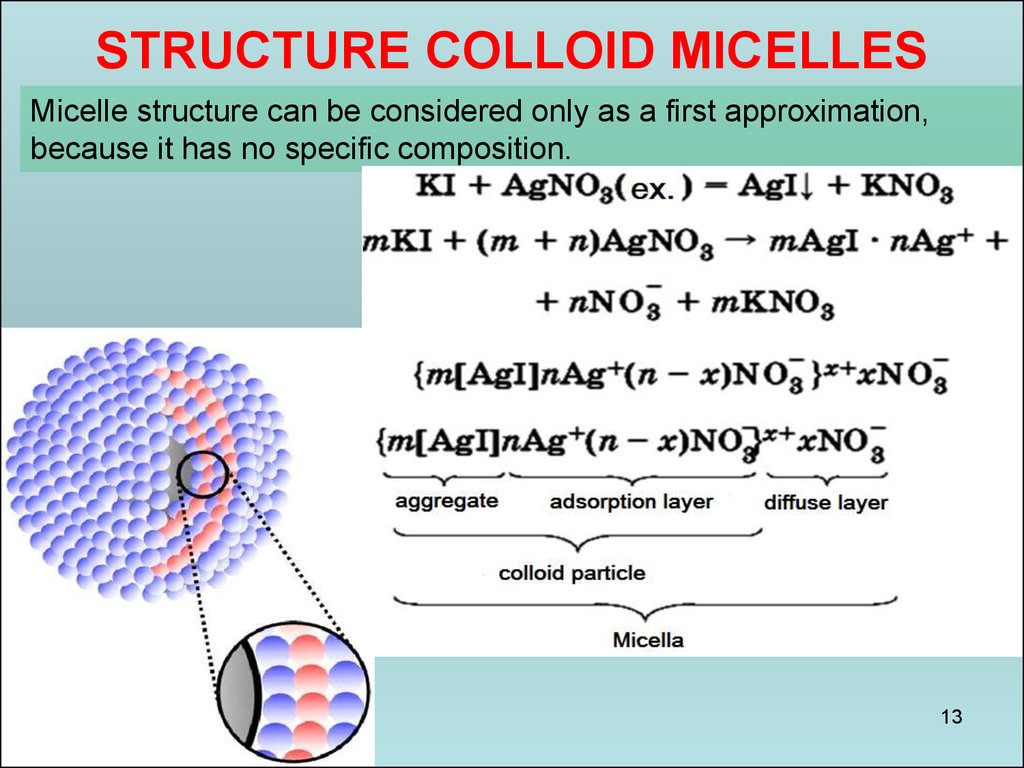
13.
STRUCTURE COLLOID MICELLESMicelle structure can be considered only as a first approximation,
because it has no specific composition.
13
14.
With an excess of onereactant
microchip
adsorbs its ions, which
I
do
not
form
a
precipitate.
As a result of this
microchip acquires a
charge, ions, informing
him that the charge Charged core attracts ions from the solution
potential-called, and he with the opposite charge - counterions;
charged crystal - core interfacial electrical double layer is formed.
micelles.
Some part of counterions adsorbed on the surface of the nucleus, forming a so-called
adsorption layer counterions; nucleus together with adsorbed thereon are called counterions
colloidal particles or granules. The remaining counter, the number of which is determined on
the basis of the rules of electrical micelles constitute a diffuse layer of counterions;
counterions adsorption and diffusion layers are in a state of dynamic equilibrium adsorption
14
- desorption.
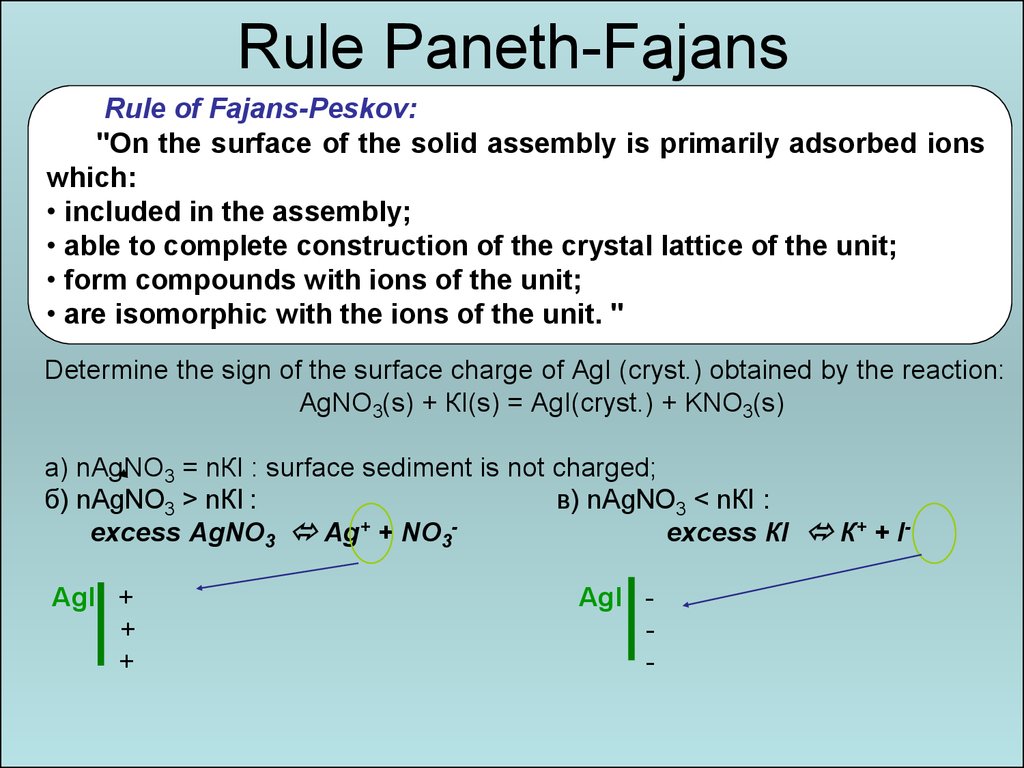
15. Rule Paneth-Fajans
Rule of Fajans-Peskov:"On the surface of the solid assembly is primarily adsorbed ions
which:
• included in the assembly;
• able to complete construction of the crystal lattice of the unit;
• form compounds with ions of the unit;
• are isomorphic with the ions of the unit. "
Determine the sign of the surface charge of AgI (cryst.) obtained by the reaction:
АgNО3(s) + КI(s) = АgI(cryst.) + KNO3(s)
а) nАgNО3 = nКI : surface sediment is not charged;
б) nАgNO3 > nКI :
в) nАgNО3 < nКI :
excess АgNO3 Аg+ + NО3excess КI К+ + IАgI +
+
+
АgI -
16.
Chemical condensation methodsMethods based on the formation of poorly soluble compounds by
chemical reactions.
1. Reduction reaction.
Recovery of sodium aurate by formaldehyde.
2NaAuO2 + 3HCOH + Na2CO3 = 2Au + 3HCOONa +NaHCO3 + H2O
Micelle structure :
m Au nAuO ( n x )Na
2
x
xNa
16
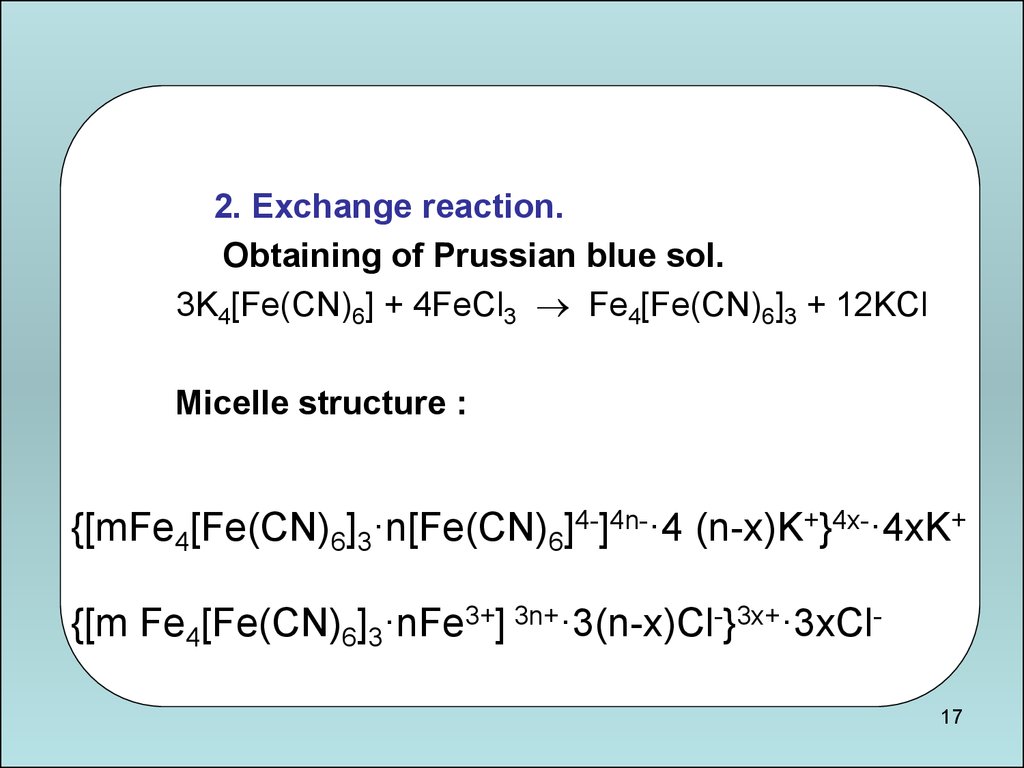
17.
2. Exchange reaction.Obtaining of Prussian blue sol.
3K4[Fe(CN)6] + 4FeCl3 Fe4[Fe(CN)6]3 + 12KCl
Micelle structure :
{[mFe4[Fe(CN)6]3·n[Fe(CN)6]4-]4n-·4 (n-х)K+}4x-·4xK+
{[m Fe4[Fe(CN)6]3·nFe3+] 3n+·3(n-х)Сl-}3x+·3xCl17
18.
2. Exchange reaction.Obtaining of silver iodide sol.
AgNO3 + KJ(exc.) = AgJ↓ + KNO3
Micelle structure :
m AgJ nJ
( n x )K
x
xK
18
19.
3. Oxidation reactionFormation sulfur sol.
2H2Sр-р + O2 = 2S ↓+ 2H2O
Micelle structure :
m S nHS
( n x )H
x
xH
19
20.
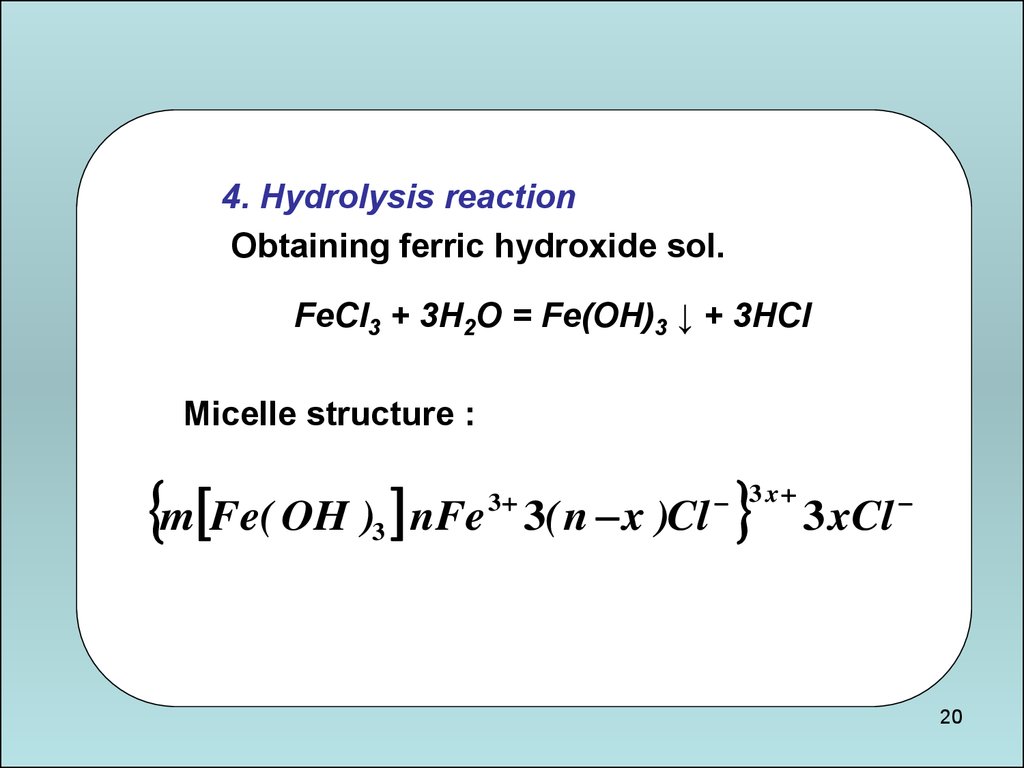
4. Hydrolysis reactionObtaining ferric hydroxide sol.
FeCl3 + 3H2O = Fe(OH)3 ↓ + 3HCl
Micelle structure :
m Fe( OH ) nFe
3
3
3( n x )Cl
3 x
3 xCl
20
21.
Peptization methodPeptization - a method based on transferring a
colloid
precipitation
primary
dimensions
are
dimensions which are highly dispersed systems.
The essence of the method: freshly fallen loose
sediment is converted into sol by treatment peptizing
agents.
21
22.
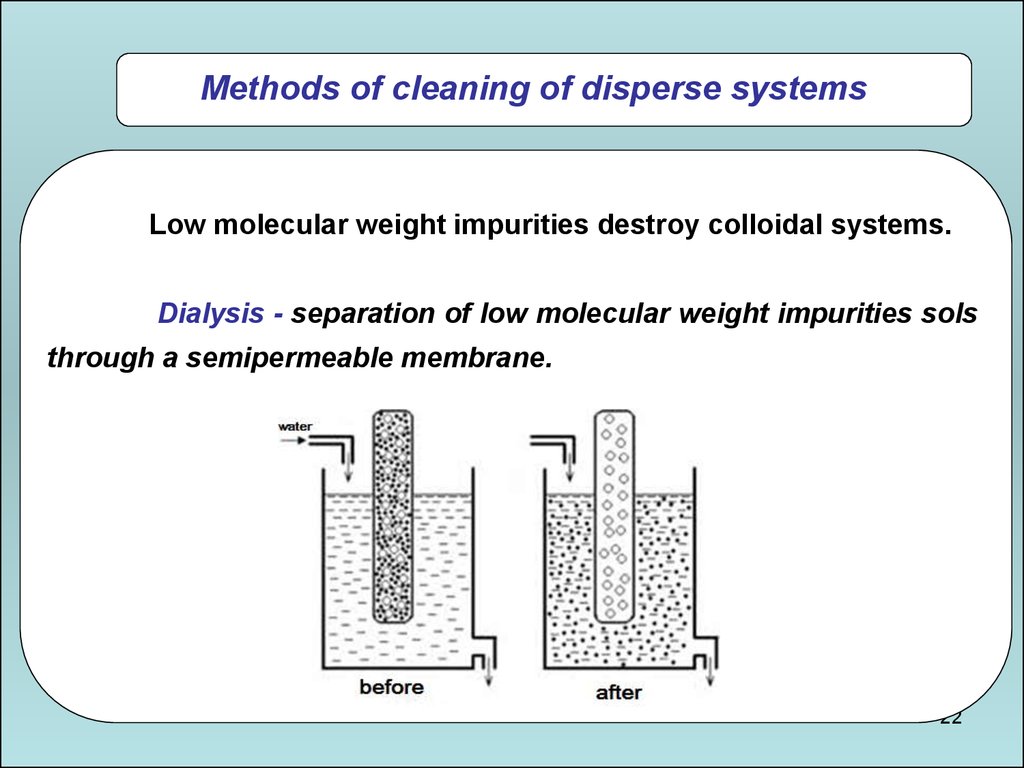
Methods of cleaning of disperse systemsLow molecular weight impurities destroy colloidal systems.
Dialysis - separation of low molecular weight impurities sols
through a semipermeable membrane.
22
23.
Methods for cleaning of disperse systemsLow molecular weight impurities destroy colloidal systems.
Electrodialysis - dialysis, accelerated by an external electric
field.
Desalination by electrodialysis.
Under the action of electric current salt
ions begin to move: positive –
The cathode to the anode and the negative
23
24.
Methods for cleaning of disperse systemsLow molecular weight impurities destroy colloidal systems.
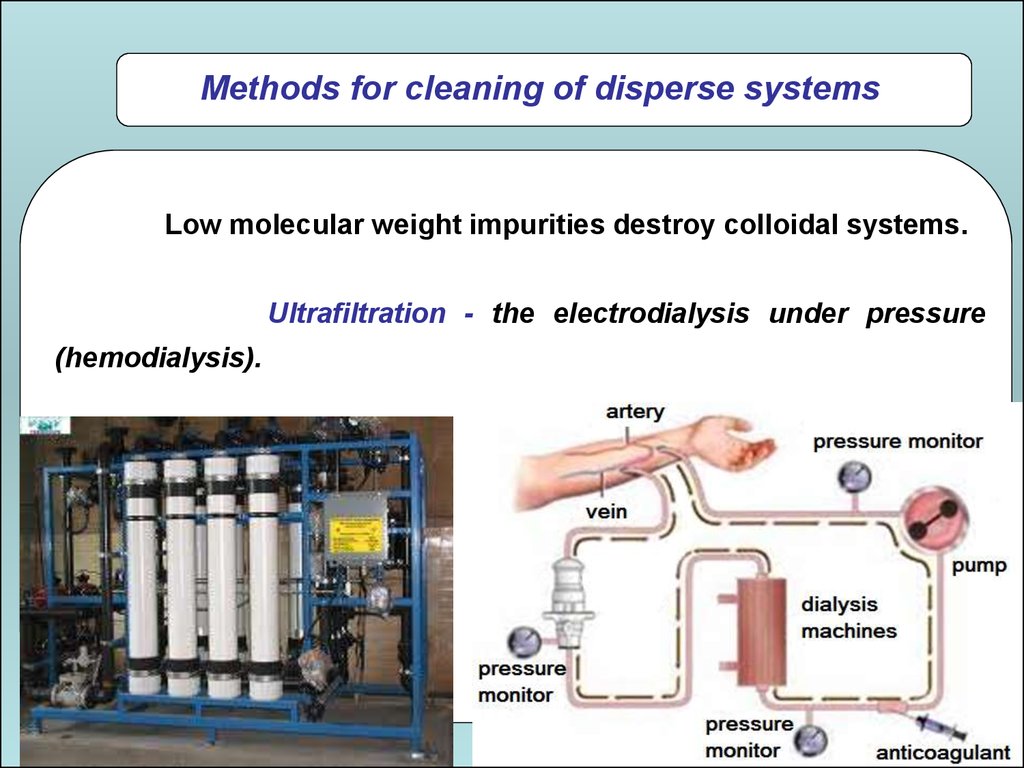
Ultrafiltration - the electrodialysis under pressure
(hemodialysis).
24
25. Molecular-kinetic properties of dispersion systems
Zaporozhye statemedical University
Department of physical and
colloid chemistry
Molecular-kinetic
properties of dispersion
systems
25
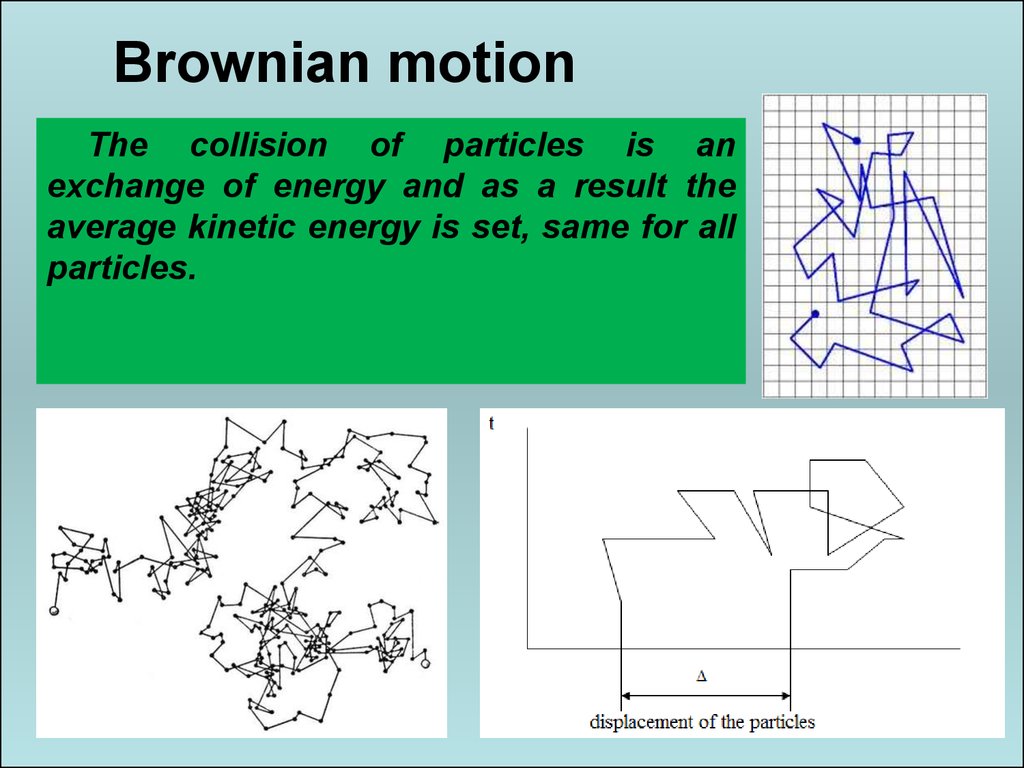
26. Brownian motion
Colloidalparticles
by
molecular-kinetic
properties are not fundamentally different from true
solutions. Weighted particles in the solution are in
constant random thermal motion.
26
27. Brownian motion
The collision of particles is anexchange of energy and as a result the
average kinetic energy is set, same for all
particles.
27
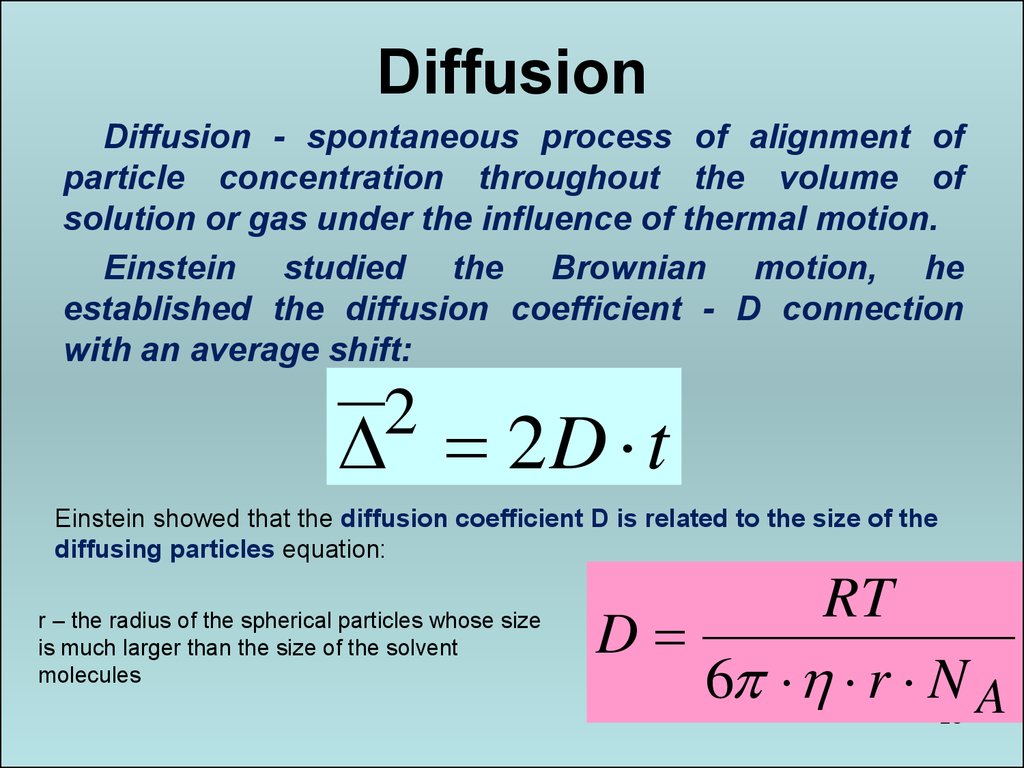
28. Diffusion
Diffusion - spontaneous process of alignment ofparticle concentration throughout the volume of
solution or gas under the influence of thermal motion.
Einstein studied the Brownian motion, he
established the diffusion coefficient - D connection
with an average shift:
2
2D t
Einstein showed that the diffusion coefficient D is related to the size of the
diffusing particles equation:
r – the radius of the spherical particles whose size
is much larger than the size of the solvent
molecules
RT
D
6 r N A
28
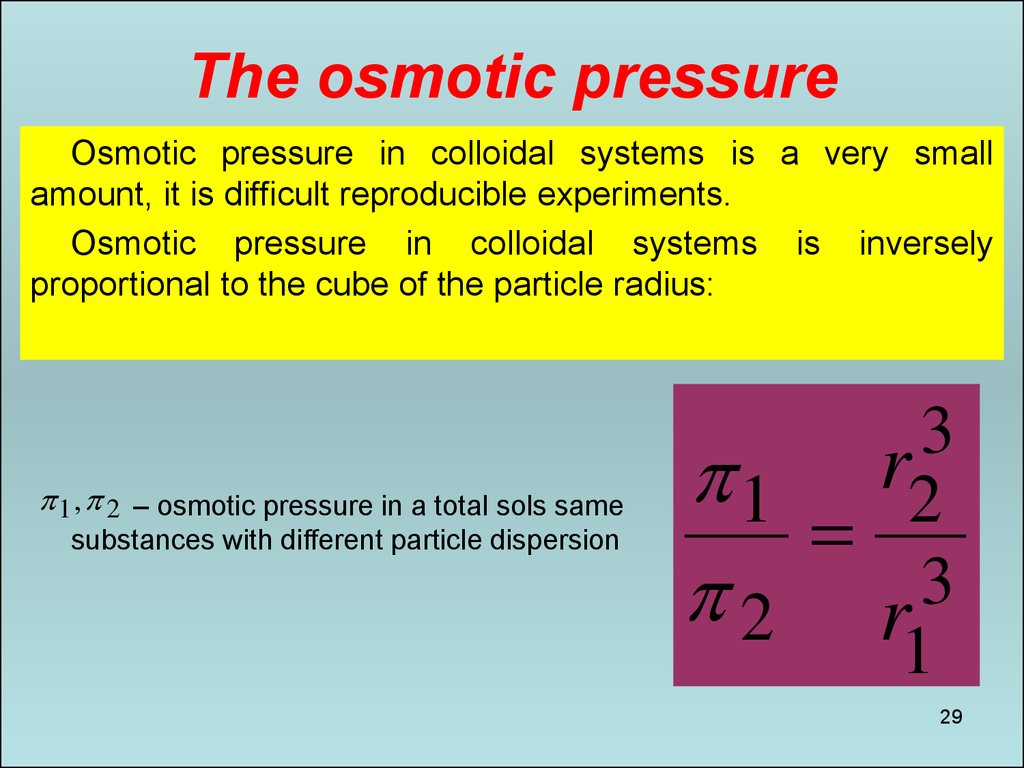
29. The osmotic pressure
Osmotic pressure in colloidal systems is a very smallamount, it is difficult reproducible experiments.
Osmotic pressure in colloidal systems is inversely
proportional to the cube of the particle radius:
1 , 2 – osmotic pressure in a total sols same
substances with different particle dispersion
3
1 r2
2 r3
1
29
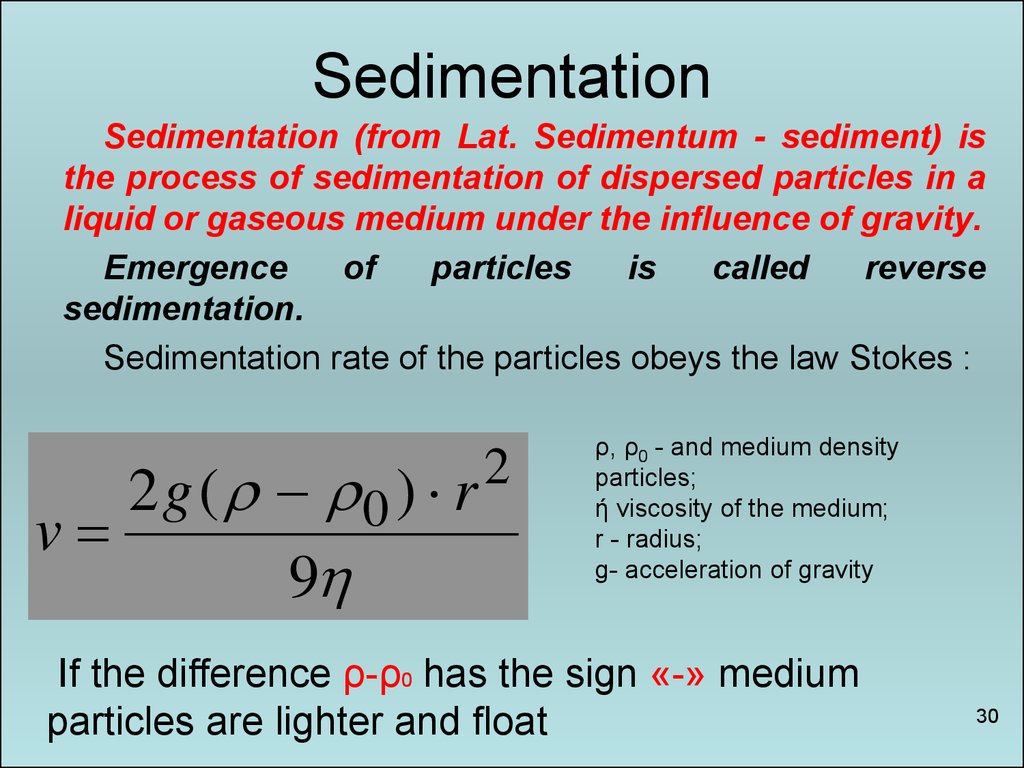
30. Sedimentation
Sedimentation (from Lat. Sedimentum - sediment) isthe process of sedimentation of dispersed particles in a
liquid or gaseous medium under the influence of gravity.
Emergence
of
particles
is
called
reverse
sedimentation.
Sedimentation rate of the particles obeys the law Stokes :
2g ( 0 ) r
v
9
2
ρ, ρ0 - and medium density
particles;
ή viscosity of the medium;
r - radius;
g- acceleration of gravity
If the difference ρ-ρ0 has the sign «-» medium
particles are lighter and float
30
31. Sedimentation analysis
For sedimentation analysis of kineticallystable systems to determine the size and
mass of the particles is not enough force
gravity.
Russian scientist AV Dumanskiy (1912)
proposed to expose colloidal systems
centrifugation.
Swedberg (1923) developed a special
centrifuge with great speed, called the
ultracentrifuge.
31
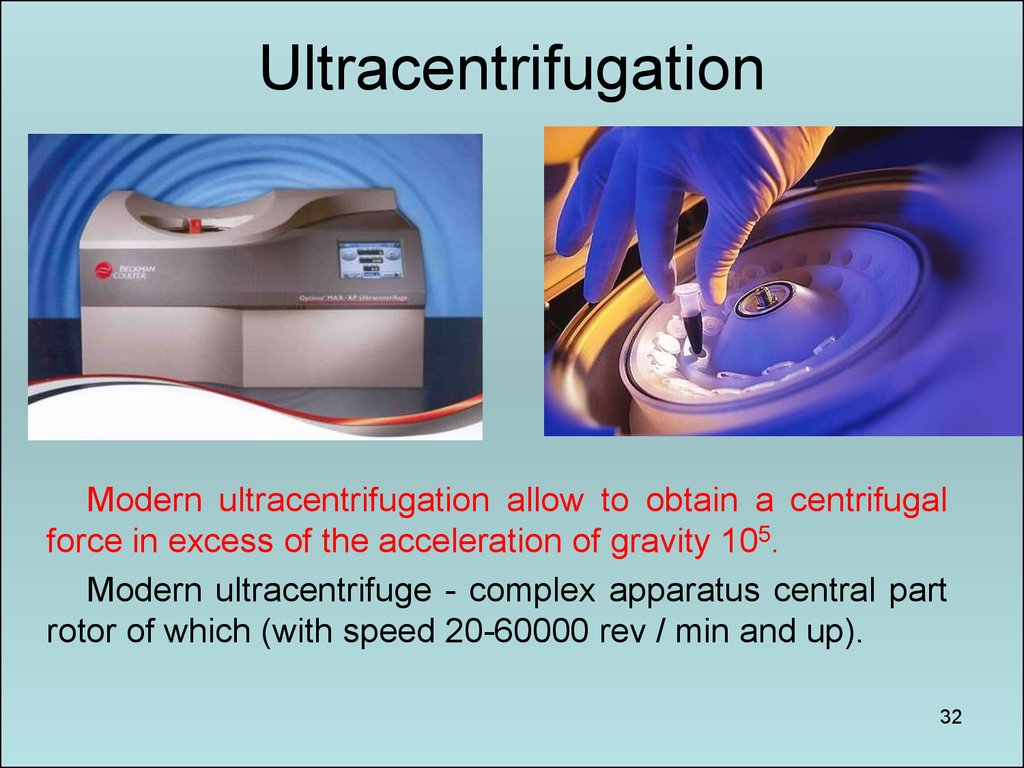
32. Ultracentrifugation
Modern ultracentrifugation allow to obtain a centrifugalforce in excess of the acceleration of gravity 105.
Modern ultracentrifuge - complex apparatus central part
rotor of which (with speed 20-60000 rev / min and up).
32
33. Optical properties of disperse systems
Zaporozhye statemedical University
Department of physical and
colloid chemistry
Optical properties of
disperse systems
33
34. The scattering of light
FaradayTyndall
This is the most characteristic optical property of colloidal systems.
The light is scattered in all directions.
This phenomenon was observed Faraday (1857) in the study of
gold sol. The phenomenon Tyndall in 1868.
Through pure liquids and molecular solutions light just
passes.
Through colloidal dispersions light ray meeting on the way a
particle is not reflected, as if it skirts, and rejected several
changes its direction (diffraction).
34
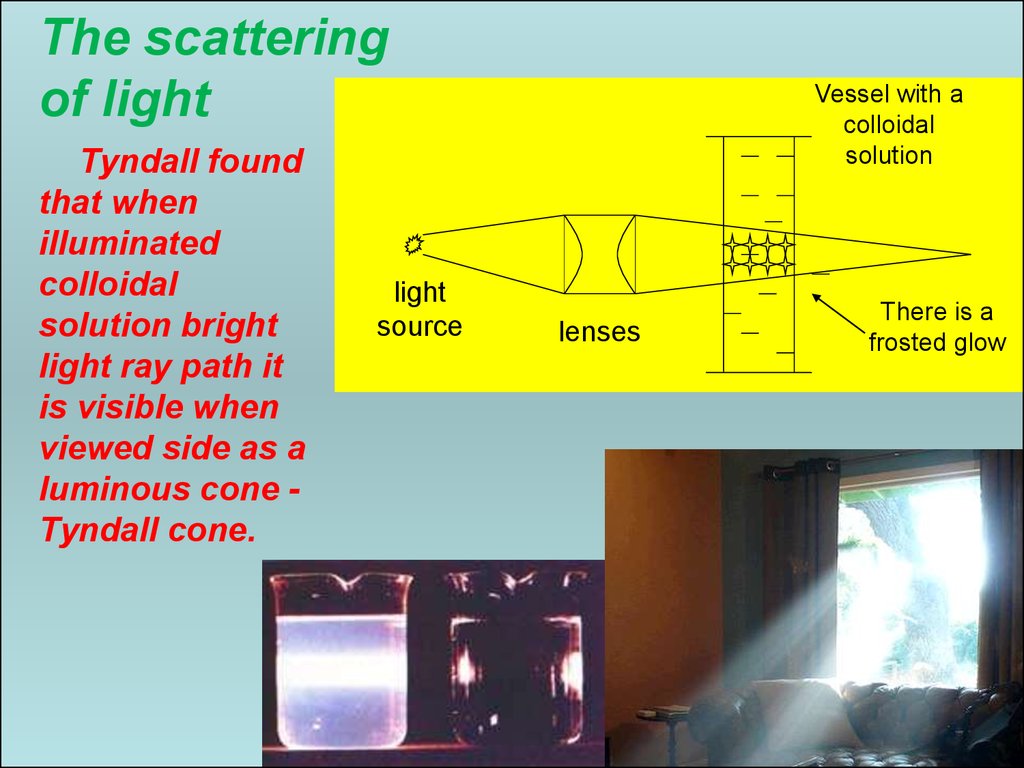
35. The scattering of light
Tyndall foundthat when
illuminated
colloidal
solution bright
light ray path it
is visible when
viewed side as a
luminous cone Tyndall cone.
light
source
Vessel with a
colloidal
solution
lenses
There is a
frosted glow
35
36. Electrical properties of disperse systems
Zaporozhye statemedical University
Department of physical and
colloid chemistry
Electrical properties of
disperse systems
36
37. DEL. Formation of a double electric layer
DEL existence of ions and the potential jump at the interface of the twophases plays an important role in many phenomena important for theory and
practice .
These include: the electrode processes , electrocapillary and electrokinetic
phenomena , phenomena associated with the electrostatic interaction of
colloidal particles , largely determine the stability of the dispersed system .
All these phenomena are interconnected through DEL , called
Electrosurface .
There are three possible mechanisms for the formation of DEL :
- Due to the transition of electrons or ions from one phase to another ( 1st
variant );
- As a result of the selective adsorption of ions in the electrolyte interphase
layer ( 2nd variant );
- As a result of the orientation of the polar molecules conjugated phases in
their interaction ( third variant ) .
37
38.
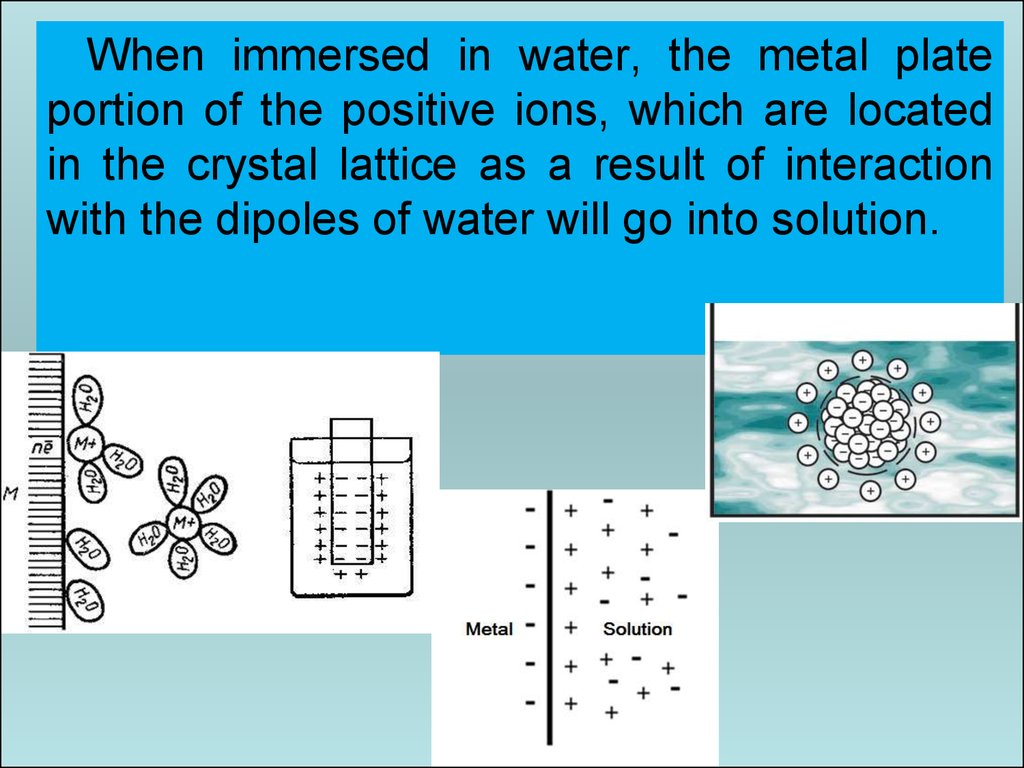
When immersed in water, the metal plateportion of the positive ions, which are located
in the crystal lattice as a result of interaction
with the dipoles of water will go into solution.
39. Electric double layer 2nd version. In the formation of AgI sol by reaction between AgNO3 and KI at AgI microcrystals adsorbed ions (Ag +, I-). If an excess of silver nitrate, the silver ions are adsorbed. When this solid phase is positively charged (varia
Electric double layer 2nd version. In the formationof AgI sol by reaction between AgNO3 and KI at
AgI microcrystals adsorbed ions (Ag +, I-). If an
excess of silver nitrate, the silver ions are
adsorbed. When this solid phase is positively
charged (variant b). Excess anions NO3-ions are
attracted to the Ag +
S
a
l
t
S
a
l
t
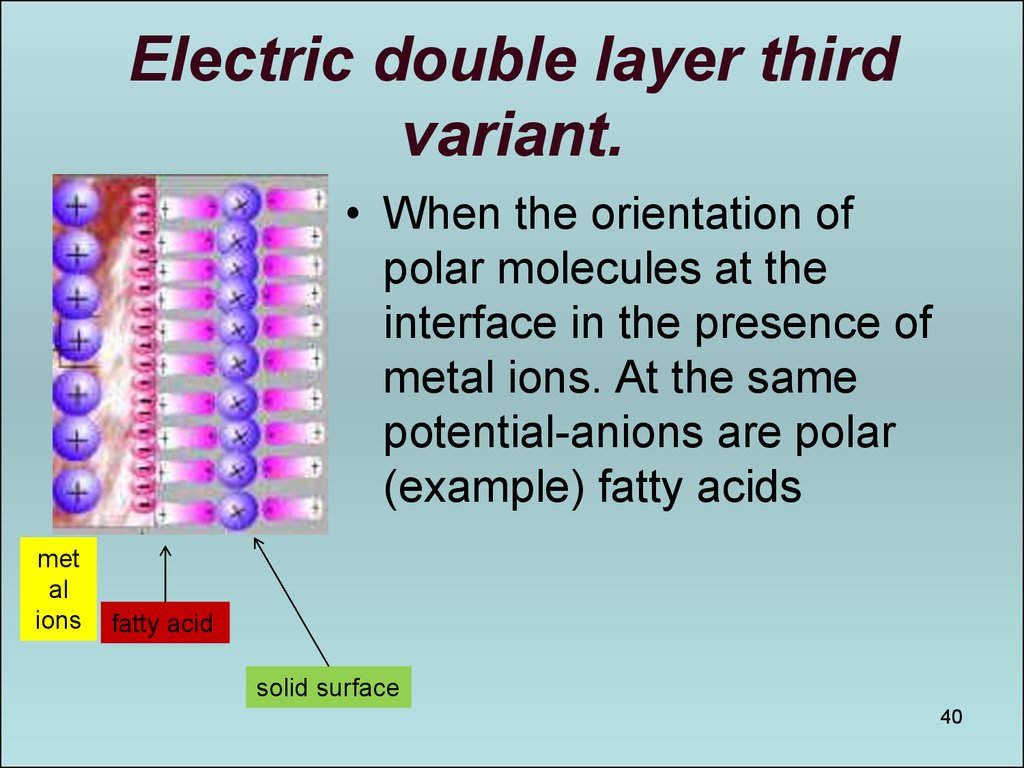
40. Electric double layer third variant.
• When the orientation ofpolar molecules at the
interface in the presence of
metal ions. At the same
potential-anions are polar
(example) fatty acids
met
al
ions
fatty acid
solid surface
40
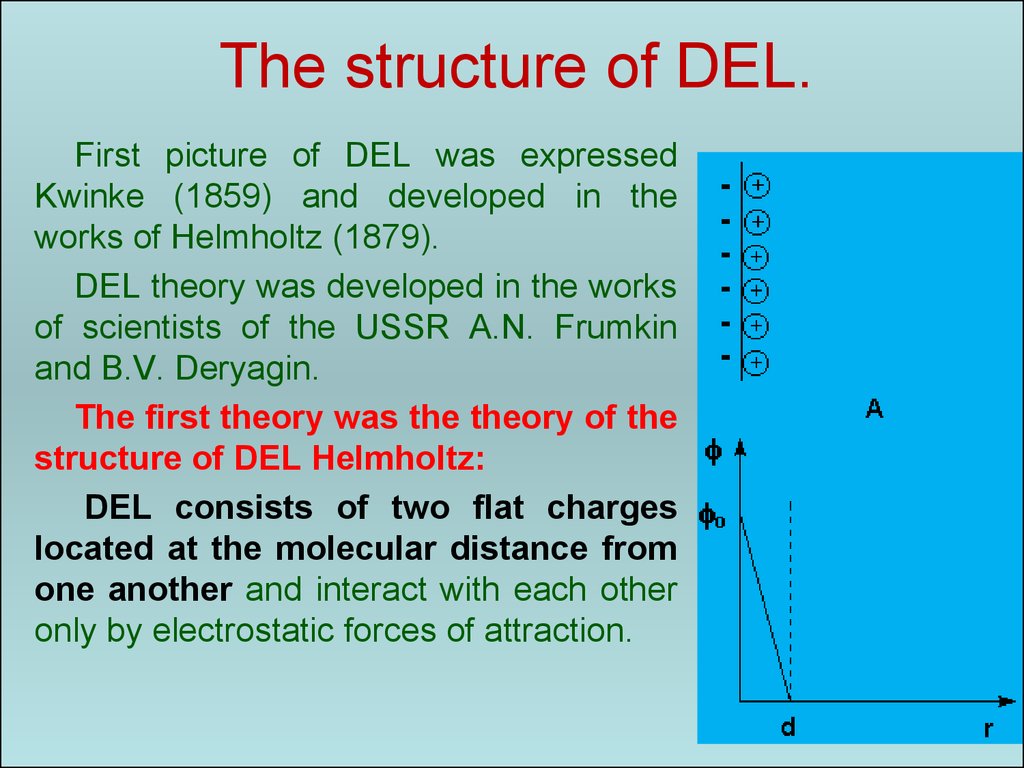
41. The structure of DEL.
First picture of DEL was expressedKwinke (1859) and developed in the
works of Helmholtz (1879).
DEL theory was developed in the works
of scientists of the USSR A.N. Frumkin
and B.V. Deryagin.
The first theory was the theory of the
structure of DEL Helmholtz:
DEL consists of two flat charges
located at the molecular distance from
one another and interact with each other
only by electrostatic forces of attraction.
41
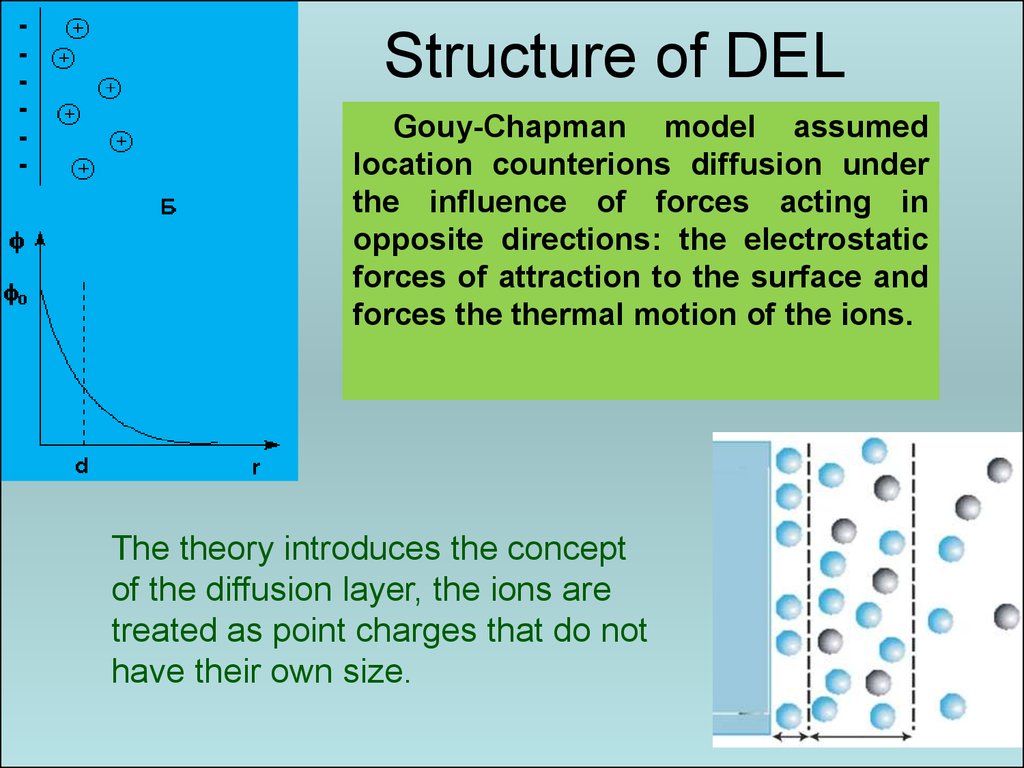
42. Structure of DEL
Gouy-Chapman model assumedlocation counterions diffusion under
the influence of forces acting in
opposite directions: the electrostatic
forces of attraction to the surface and
forces the thermal motion of the ions.
The theory introduces the concept
of the diffusion layer, the ions are
treated as point charges that do not
have their own size.
42
43. Structure of DEL
According to modern concepts (Stern’s theory)structure of DEL: ions are included in the solid
phase, form the inner lining of the double layer,
ions of opposite sign, i.e. counterions forming an
outer lining, wherein the counterions part is in
direct contact with ions of the solid phase, forming
a dense layer, and another part is counterions
diffused layer.
43
44.
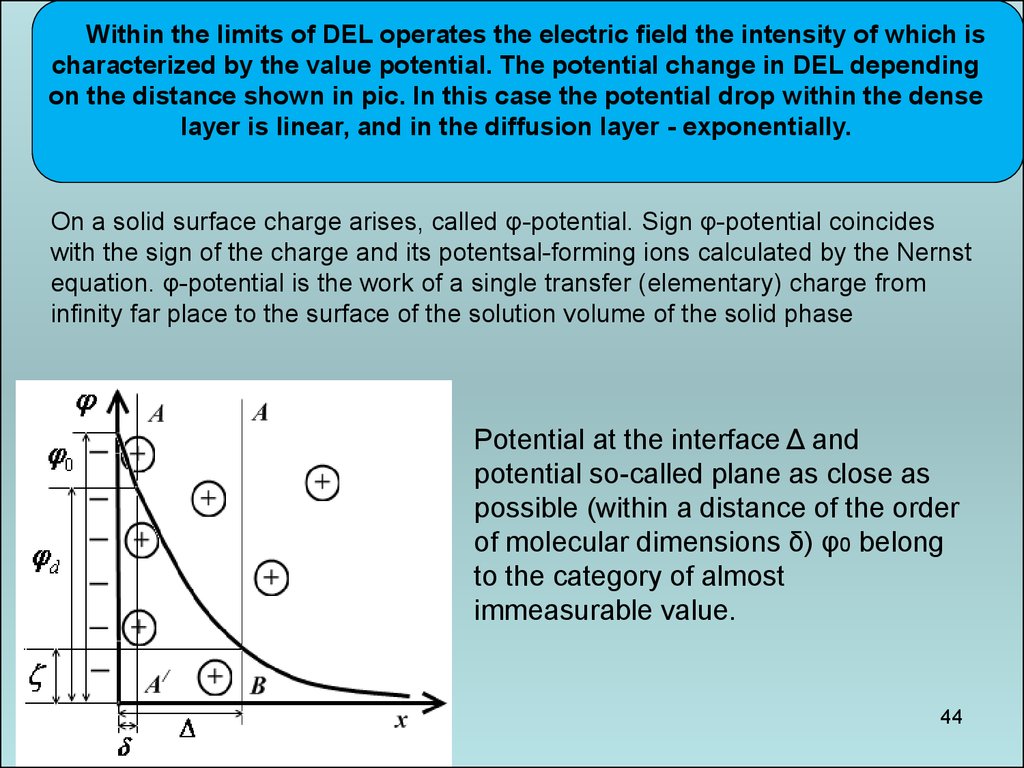
Within the limits of DEL operates the electric field the intensity of which ischaracterized by the value potential. The potential change in DEL depending
on the distance shown in pic. In this case the potential drop within the dense
layer is linear, and in the diffusion layer - exponentially.
On a solid surface charge arises, called φ-potential. Sign φ-potential coincides
with the sign of the charge and its potentsal-forming ions calculated by the Nernst
equation. φ-potential is the work of a single transfer (elementary) charge from
infinity far place to the surface of the solution volume of the solid phase
Potential at the interface Δ and
potential so-called plane as close as
possible (within a distance of the order
of molecular dimensions δ) φ0 belong
to the category of almost
immeasurable value.
44
45.
To characterize the electrical properties of the surface using ζpotential-potential boundary sliding phases determinedexperimentally by various methods. ζ-potential can be
represented as the work necessary for the transfer of charge
from the unit element of an infinitely distant volume of solution
on the sliding surface. ζ-potential sign coincides with φpotential
The electrokinetic potential (zeta potential)
- potential arising at the boundary AB
sliding phase when relative movement in
an electric field. This potential is
calculated from the experimental data for
the equation Helmholtz -Smoluchowski
U0 – velocity of the fluid, 0 – constant, - dielectric
permittivity a liquid, E – the electric field strength,
- potential, - fluid viscosity.
= *U0/ 0* *E
45
46.
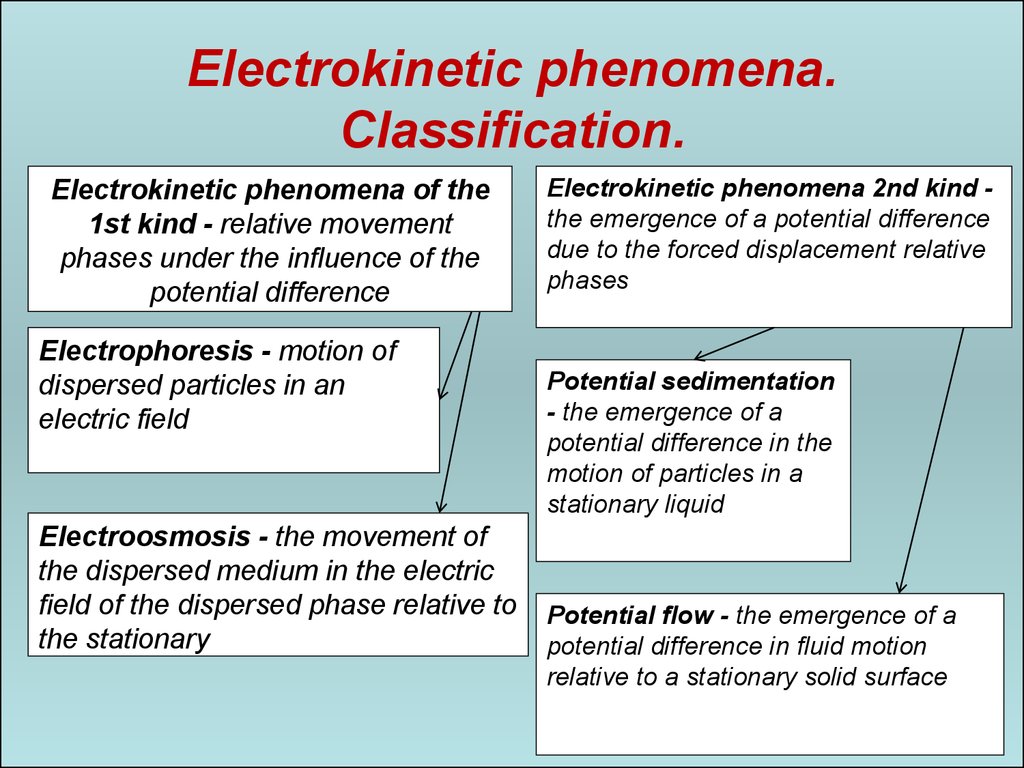
Electrokinetic phenomena.Classification.
Electrokinetic phenomena of the
1st kind - relative movement
phases under the influence of the
potential difference
Electrophoresis - motion of
dispersed particles in an
electric field
Electroosmosis - the movement of
the dispersed medium in the electric
field of the dispersed phase relative to
the stationary
Electrokinetic phenomena 2nd kind the emergence of a potential difference
due to the forced displacement relative
phases
Potential sedimentation
- the emergence of a
potential difference in the
motion of particles in a
stationary liquid
Potential flow - the emergence of a
potential difference in fluid motion
relative to a stationary solid surface
46
47.
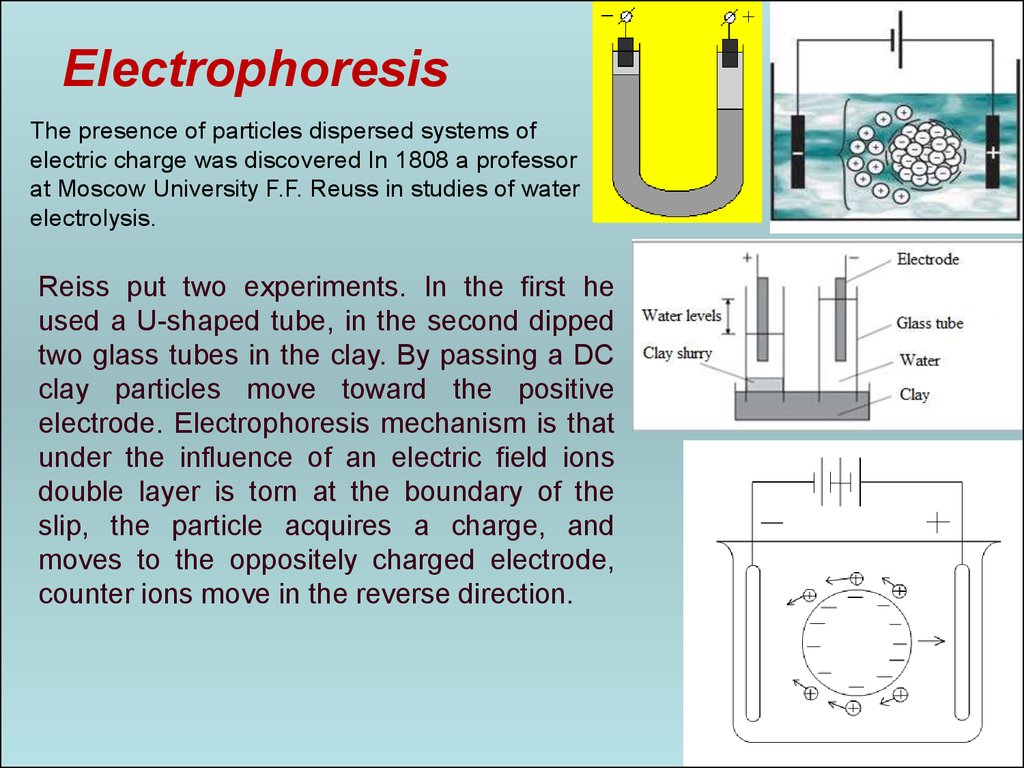
ElectrophoresisThe presence of particles dispersed systems of
electric charge was discovered In 1808 a professor
at Moscow University F.F. Reuss in studies of water
electrolysis.
Reiss put two experiments. In the first he
used a U-shaped tube, in the second dipped
two glass tubes in the clay. By passing a DC
clay particles move toward the positive
electrode. Electrophoresis mechanism is that
under the influence of an electric field ions
double layer is torn at the boundary of the
slip, the particle acquires a charge, and
moves to the oppositely charged electrode,
counter ions move in the reverse direction.
47
48.
ElectrophoresisParticle velocity of the dispersed phase
electrophoresis and speed dispersion medium
when electroosmosis directly proportional to the
electric field E and the dielectric constant ε of the
dispersion medium and inversely proportional to the
medium viscosity η. Particle velocity of the
dispersed phase electrophoresis U related to the
value ζ-potential of the equation HelmholtzSmoluchowski
U0 = 0* *E* /
Electrophoresis allows to deliver the drug directly
to the affected area and gradually establish there a
sufficient concentration.
48
49.
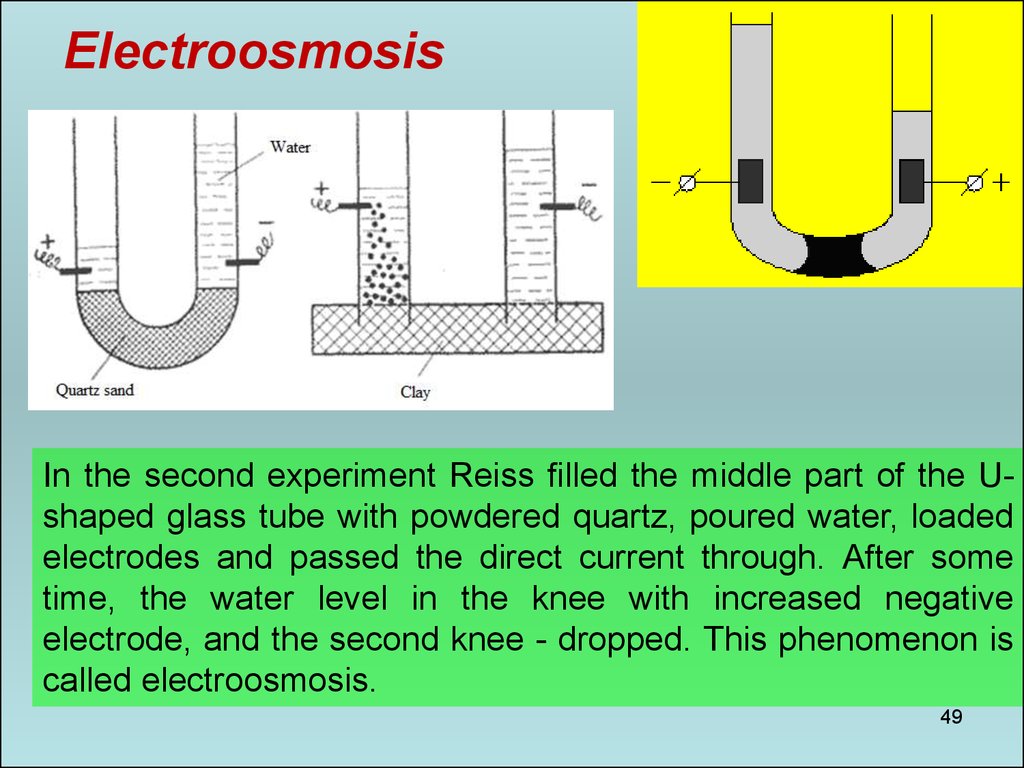
ElectroosmosisIn the second experiment Reiss filled the middle part of the Ushaped glass tube with powdered quartz, poured water, loaded
electrodes and passed the direct current through. After some
time, the water level in the knee with increased negative
electrode, and the second knee - dropped. This phenomenon is
called electroosmosis.
49
50.
Potential ofleakage and
sedimentation
Potential leakage (the effect of Kwinke) is a phenomenon of the
potential difference in the dispersion medium motion relative to
the fixed dispersion phase.
Sedimentation potential (Dorn effect) - the emergence of a
potential difference in induced motion of the dispersed phase
relative to the fixed dispersion medium.
50
51. Stability and coagulation of colloidal systems
Zaporozhye statemedical University
Department of physical and
colloid chemistry
Stability and
coagulation of
colloidal systems
51
52.
Stability of disperse systemsStability - the immutability of time the basic
parameters of the dispersed system: the degree of
dispersion and uniform distribution of the dispersed
phase in the dispersion medium.
On the suggestion of NP Peskov (1920) the stability of disperse systems
are divided into two types: Kinetic stability - property dispersed particles
held in suspension without collapsing.
Aggregate stability - the ability of the dispersed phase provide blocking
resistance and thus maintain a certain degree of dispersion of this phase
as a whole.
52
53.
Coagulation is a process of adhesion ofcolloidal particles to form larger aggregates
with consequent loss of kinetic stability, can be
caused by:
introduction of electrolytes;
heating or freezing of the dispersed system;
mechanical action;
high-frequency oscillations;
ultracentrifugation.
53
54.
Coagulation sols electrolytesПравила электролитной коагуляции
All electrolytes at certain concentrations can cause
coagulation of the sol.
•Usually
charge sign: sol coagulation that causes ion
electrolyte sign of the charge which is opposite to the charge of
the colloidal particle. This ion-ion called coagulator.
mFe OH 3 nFeCl3 Fe OH 3 m nFe 3 n x Cl
•Each
3
3x
3xCl
electrolyte in relation to the colloidal solution has a
threshold of coagulation.
54
55.
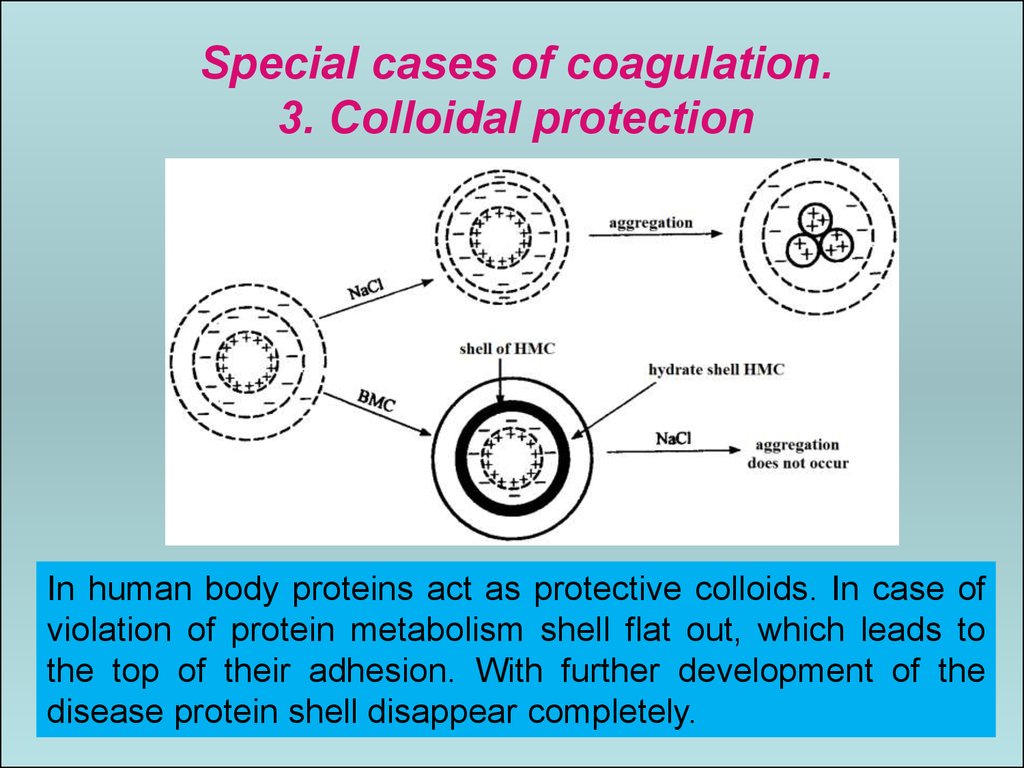
Special cases of coagulation.3. Colloidal protection
In human body proteins act as protective colloids. In case of
violation of protein metabolism shell flat out, which leads to
the top of their adhesion. With further development of the
disease protein shell disappear completely.
55

























 chemistry
chemistry








